Related Research Articles

Magnetic resonance imaging (MRI) is a medical imaging technique used in radiology to form pictures of the anatomy and the physiological processes inside the body. MRI scanners use strong magnetic fields, magnetic field gradients, and radio waves to generate images of the organs in the body. MRI does not involve X-rays or the use of ionizing radiation, which distinguishes it from computed tomography (CT) and positron emission tomography (PET) scans. MRI is a medical application of nuclear magnetic resonance (NMR) which can also be used for imaging in other NMR applications, such as NMR spectroscopy.

Functional magnetic resonance imaging or functional MRI (fMRI) measures brain activity by detecting changes associated with blood flow. This technique relies on the fact that cerebral blood flow and neuronal activation are coupled. When an area of the brain is in use, blood flow to that region also increases.

Cerebral circulation is the movement of blood through a network of cerebral arteries and veins supplying the brain. The rate of cerebral blood flow in an adult human is typically 750 milliliters per minute, or about 15% of cardiac output. Arteries deliver oxygenated blood, glucose and other nutrients to the brain. Veins carry "used or spent" blood back to the heart, to remove carbon dioxide, lactic acid, and other metabolic products. The neurovascular unit regulates cerebral blood flow so that activated neurons can be supplied with energy in the right amount and at the right time. Because the brain would quickly suffer damage from any stoppage in blood supply, the cerebral circulatory system has safeguards including autoregulation of the blood vessels. The failure of these safeguards may result in a stroke. The volume of blood in circulation is called the cerebral blood flow. Sudden intense accelerations change the gravitational forces perceived by bodies and can severely impair cerebral circulation and normal functions to the point of becoming serious life-threatening conditions.

Perfusion is the passage of fluid through the circulatory system or lymphatic system to an organ or a tissue, usually referring to the delivery of blood to a capillary bed in tissue. Perfusion may also refer to fixation via perfusion, used in histological studies. Perfusion is measured as the rate at which blood is delivered to tissue, or volume of blood per unit time per unit tissue mass. The SI unit is m3/(s·kg), although for human organs perfusion is typically reported in ml/min/g. The word is derived from the French verb perfuser, meaning to "pour over or through". All animal tissues require an adequate blood supply for health and life. Poor perfusion (malperfusion), that is, ischemia, causes health problems, as seen in cardiovascular disease, including coronary artery disease, cerebrovascular disease, peripheral artery disease, and many other conditions.

Neuroimaging is the use of quantitative (computational) techniques to study the structure and function of the central nervous system, developed as an objective way of scientifically studying the healthy human brain in a non-invasive manner. Increasingly it is also being used for quantitative research studies of brain disease and psychiatric illness. Neuroimaging is highly multidisciplinary involving neuroscience, computer science, psychology and statistics, and is not a medical specialty. Neuroimaging is sometimes confused with neuroradiology.
During nuclear magnetic resonance observations, spin–lattice relaxation is the mechanism by which the longitudinal component of the total nuclear magnetic moment vector (parallel to the constant magnetic field) exponentially relaxes from a higher energy, non-equilibrium state to thermodynamic equilibrium with its surroundings (the "lattice"). It is characterized by the spin–lattice relaxation time, a time constant known as T1.
Kenneth Kin Man Kwong is a Hong Kong-born American nuclear physicist. He is a pioneer in human brain imaging. He received his bachelor's degree in Political Science in 1972 from the University of California, Berkeley. He went on to receive his Ph.D. in physics from the University of California, Riverside studying photon-photon collision interactions.
MRI contrast agents are contrast agents used to improve the visibility of internal body structures in magnetic resonance imaging (MRI). The most commonly used compounds for contrast enhancement are gadolinium-based contrast agents (GBCAs). Such MRI contrast agents shorten the relaxation times of nuclei within body tissues following oral or intravenous administration.

Cardiac magnetic resonance imaging, also known as cardiovascular MRI, is a magnetic resonance imaging (MRI) technology used for non-invasive assessment of the function and structure of the cardiovascular system. Conditions in which it is performed include congenital heart disease, cardiomyopathies and valvular heart disease, diseases of the aorta such as dissection, aneurysm and coarctation, coronary heart disease. It can also be used to look at pulmonary veins. Patient information may be found here.
Perfusion is the passage of fluid through the lymphatic system or blood vessels to an organ or a tissue. The practice of perfusion scanning is the process by which this perfusion can be observed, recorded and quantified. The term perfusion scanning encompasses a wide range of medical imaging modalities.

Magnetic resonance imaging (MRI) is a medical imaging technique mostly used in radiology and nuclear medicine in order to investigate the anatomy and physiology of the body, and to detect pathologies including tumors, inflammation, neurological conditions such as stroke, disorders of muscles and joints, and abnormalities in the heart and blood vessels among others. Contrast agents may be injected intravenously or into a joint to enhance the image and facilitate diagnosis. Unlike CT and X-ray, MRI uses no ionizing radiation and is, therefore, a safe procedure suitable for diagnosis in children and repeated runs. Patients with specific non-ferromagnetic metal implants, cochlear implants, and cardiac pacemakers nowadays may also have an MRI in spite of effects of the strong magnetic fields. This does not apply on older devices, and details for medical professionals are provided by the device's manufacturer.

Magnetic resonance imaging of the brain uses magnetic resonance imaging (MRI) to produce high quality two-dimensional or three-dimensional images of the brain and brainstem as well as the cerebellum without the use of ionizing radiation (X-rays) or radioactive tracers.
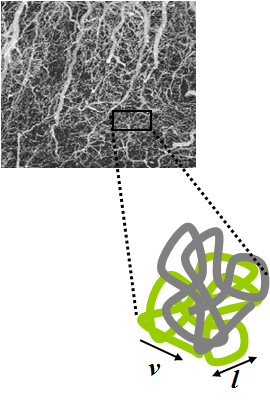
Intravoxel incoherent motion (IVIM) imaging is a concept and a method initially introduced and developed by Le Bihan et al. to quantitatively assess all the microscopic translational motions that could contribute to the signal acquired with diffusion MRI. In this model, biological tissue contains two distinct environments: molecular diffusion of water in the tissue, and microcirculation of blood in the capillary network (perfusion). The concept introduced by D. Le Bihan is that water flowing in capillaries mimics a random walk (Fig.1), as long as the assumption that all directions are represented in the capillaries is satisfied.

Resting state fMRI is a method of functional magnetic resonance imaging (fMRI) that is used in brain mapping to evaluate regional interactions that occur in a resting or task-negative state, when an explicit task is not being performed. A number of resting-state brain networks have been identified, one of which is the default mode network. These brain networks are observed through changes in blood flow in the brain which creates what is referred to as a blood-oxygen-level dependent (BOLD) signal that can be measured using fMRI.

Perfusion MRI or perfusion-weighted imaging (PWI) is perfusion scanning by the use of a particular MRI sequence. The acquired data are then post-processed to obtain perfusion maps with different parameters, such as BV, BF, MTT and TTP.

CONN is a Matlab-based cross-platform imaging software for the computation, display, and analysis of functional connectivity in fMRI in the resting state and during task.
Synthetic MRI is a simulation method in Magnetic Resonance Imaging (MRI), for generating contrast weighted images based on measurement of tissue properties. The synthetic (simulated) images are generated after an MR study, from parametric maps of tissue properties. It is thereby possible to generate several contrast weightings from the same acquisition. This is different from conventional MRI, where the signal acquired from the tissue is used to generate an image directly, often generating only one contrast weighting per acquisition. The synthetic images are similar in appearance to those normally acquired with an MRI scanner.
The history of magnetic resonance imaging (MRI) includes the work of many researchers who contributed to the discovery of nuclear magnetic resonance (NMR) and described the underlying physics of magnetic resonance imaging, starting early in the twentieth century. One researcher was American physicist Isidor Isaac Rabi who won the Nobel Prize in Physics in 1944 for his discovery of nuclear magnetic resonance, which is used in magnetic resonance imaging. MR imaging was invented by Paul C. Lauterbur who developed a mechanism to encode spatial information into an NMR signal using magnetic field gradients in September 1971; he published the theory behind it in March 1973.

An MRI pulse sequence in magnetic resonance imaging (MRI) is a particular setting of pulse sequences and pulsed field gradients, resulting in a particular image appearance.
Magnetic resonance fingerprinting (MRF) is methodology in quantitative magnetic resonance imaging (MRI) characterized by a pseudo-randomized acquisition strategy. It involves creating unique signal patterns or 'fingerprints' for different materials or tissues after which a pattern recognition algorithm matches these fingerprints with a predefined dictionary of expected signal patterns. This process translates the data into quantitative maps, revealing information about the magnetic properties being investigated.
References
- ↑ Fortin F, Gaillard F. "Arterial spin labelling (ASL) MR perfusion". Radiopaedia . Retrieved 2017-10-15.
- ↑ "Arterial spin labeling". University of Michigan . Retrieved 2017-10-27.
- ↑ Williams, D. S.; Detre, J. A.; Leigh, J. S.; Koretsky, A. P. (1992-01-01). "Magnetic resonance imaging of perfusion using spin inversion of arterial water". Proceedings of the National Academy of Sciences. 89 (1): 212–216. Bibcode:1992PNAS...89..212W. doi: 10.1073/pnas.89.1.212 . ISSN 0027-8424. PMC 48206 . PMID 1729691.
- ↑ Detre, John A.; Leigh, John S.; Williams, Donald S.; Koretsky, Alan P. (January 1992). "Perfusion imaging". Magnetic Resonance in Medicine. 23 (1): 37–45. doi:10.1002/mrm.1910230106. ISSN 0740-3194. PMID 1734182. S2CID 260421572.
- ↑ Leigh, J.S., Detre, J.A., Williams, D.S., Koretsky, A.P. "Methods for measuring perfusion using magnetic resonance imaging" US Patent No. 5,402,785 (1995).
- ↑ Koretsky AP (August 2012). "Early development of arterial spin labeling to measure regional brain blood flow by MRI". NeuroImage. 62 (2): 602–7. doi:10.1016/j.neuroimage.2012.01.005. PMC 4199083 . PMID 22245338.
- ↑ Nederveen, Aart J.; Smits, Marion; Majoie, Charles B. L. M.; Osch, Matthias J. P. van; Kuijer, Joost P. A.; Heijtel, Dennis F. R.; Steketee, Rebecca M. E.; Mutsaerts, Henri J. M. M. (2014-08-04). "Inter-Vendor Reproducibility of Pseudo-Continuous Arterial Spin Labeling at 3 Tesla". PLOS ONE. 9 (8): e104108. Bibcode:2014PLoSO...9j4108M. doi: 10.1371/journal.pone.0104108 . ISSN 1932-6203. PMC 4121318 . PMID 25090654.
- ↑ Alsop, David C.; Detre, John A.; Golay, Xavier; Günther, Matthias; Hendrikse, Jeroen; Hernandez-Garcia, Luis; Lu, Hanzhang; MacIntosh, Bradley J.; Parkes, Laura M. (January 2015). "Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia". Magnetic Resonance in Medicine. 73 (1): 102–116. doi:10.1002/mrm.25197. ISSN 1522-2594. PMC 4190138 . PMID 24715426.
- ↑ Schmid, Sophie; Heijtel, Dennis F. R.; Mutsaerts, Henri J. M. M.; Boellaard, Ronald; Lammertsma, Adriaan A.; Nederveen, Aart J.; van Osch, Matthias J. P. (August 2015). "Comparison of velocity- and acceleration-selective arterial spin labeling with [15O]H2O positron emission tomography". Journal of Cerebral Blood Flow & Metabolism. 35 (8): 1296–1303. doi:10.1038/jcbfm.2015.42. ISSN 1559-7016. PMC 4528003 . PMID 25785831.
- ↑ Shao, Xingfeng; Jann, Kay; Ma, Samantha J.; Yan, Lirong; Montagne, Axel; Ringman, John M.; Zlokovic, Berislav V.; Wang, Danny J. J. (30 November 2020). "Comparison Between Blood-Brain Barrier Water Exchange Rate and Permeability to Gadolinium-Based Contrast Agent in an Elderly Cohort". Frontiers in Neuroscience. 14. doi: 10.3389/fnins.2020.571480 . PMC 7733970 . PMID 33328848.
- ↑ Shao, Xingfeng; Ma, Samantha J.; Casey, Marlene; D’Orazio, Lina; Ringman, John M.; Wang, Danny J.J. (May 2019). "Mapping water exchange across the blood–brain barrier using 3D diffusion-prepared arterial spin labeled perfusion MRI". Magnetic Resonance in Medicine. 81 (5): 3065–3079. doi:10.1002/mrm.27632. PMC 6414249 . PMID 30561821.
- ↑ Mouchtouris, Nikolaos; Ailes, Isaiah; Gooch, Reid; Raimondo, Christian; Oghli, Yazan Shamli; Tjoumakaris, Stavropoula; Jabbour, Pascal; Rosenwasser, Robert; Alizadeh, Mahdi (June 2024). "Quantifying blood-brain barrier permeability in patients with ischemic stroke using non-contrast MRI". Magnetic Resonance Imaging. 109: 165–172. doi:10.1016/j.mri.2024.03.027. PMID 38513785.
- ↑ Ling, Chen; Zhang, Jinyuan; Shao, Xingfeng; Bai, Li; Li, Zhixin; Sun, Yunchuang; Li, Fan; Wang, Zhaoxia; Xue, Rong; Zhuo, Yan; Yang, Qi; Zhang, Zihao; Wang, Danny J. J.; Yuan, Yun (26 April 2023). "Diffusion prepared pseudo-continuous arterial spin labeling reveals blood–brain barrier dysfunction in patients with CADASIL". European Radiology. 33 (10): 6959–6969. doi:10.1007/s00330-023-09652-7. PMC 10567537. PMID 37099178.
- ↑ Li, Yingying; Ying, Yunqing; Yao, Tingyan; Jia, Xuejia; Liang, Huilou; Tang, Weijun; Jia, Xiuqin; Song, Haiqing; Shao, Xingfeng; Wang, Danny J J; Wang, Chaodong; Cheng, Xin; Yang, Qi (3 July 2023). "Decreased water exchange rate across blood–brain barrier in hereditary cerebral small vessel disease". Brain. 146 (7): 3079–3087. doi:10.1093/brain/awac500. PMC 10316759 . PMID 36625892.
- ↑ Tiwari, YV; Lu, J; Shen, Q; Cerqueira, B; Duong, TQ (August 2017). "Magnetic resonance imaging of blood-brain barrier permeability in ischemic stroke using diffusion-weighted arterial spin labeling in rats". Journal of Cerebral Blood Flow and Metabolism. 37 (8): 2706–2715. doi:10.1177/0271678X16673385. PMC 5536782 . PMID 27742887.
- ↑ Wang, Ze; Aguirre, Geoffrey K.; Rao, Hengyi; Wang, Jiongjiong; Fernández-Seara, María A.; Childress, Anna R.; Detre, John A. (February 2008). "Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx". Magnetic Resonance Imaging. 26 (2): 261–269. doi:10.1016/j.mri.2007.07.003. ISSN 0730-725X. PMC 2268990 . PMID 17826940.
- ↑ Chen, J. Jean; Jann, Kay; Wang, Danny J.J. (2015-11-01). "Characterizing Resting-State Brain Function Using Arterial Spin Labeling". Brain Connectivity. 5 (9): 527–542. doi:10.1089/brain.2015.0344. ISSN 2158-0014. PMC 4652156 . PMID 26106930.
- ↑ Jann, Kay; Orosz, Ariane; Dierks, Thomas; Wang, Danny J. J.; Wiest, Roland; Federspiel, Andrea (2013-10-01). "Quantification of Network Perfusion in ASL Cerebral Blood Flow Data with Seed Based and ICA Approaches" (PDF). Brain Topography. 26 (4): 569–580. doi:10.1007/s10548-013-0280-3. ISSN 1573-6792. PMID 23508714. S2CID 1359908.
- ↑ Chen F, Ni YC (March 2012). "Magnetic resonance diffusion-perfusion mismatch in acute ischemic stroke: An update". World Journal of Radiology. 4 (3): 63–74. doi: 10.4329/wjr.v4.i3.63 . PMC 3314930 . PMID 22468186.
- ↑ Grade M, Hernandez Tamames JA, Pizzini FB, Achten E, Golay X, Smits M (December 2015). "A neuroradiologist's guide to arterial spin labeling MRI in clinical practice". Neuroradiology. 57 (12): 1181–202. doi:10.1007/s00234-015-1571-z. PMC 4648972 . PMID 26351201.
- ↑ Mouchtouris, N; Ailes, I; Gooch, R; Raimondo, C; Oghli, YS; Tjoumakaris, S; Jabbour, P; Rosenwasser, R; Alizadeh, M (19 March 2024). "Quantifying blood-brain barrier permeability in patients with ischemic stroke using non-contrast MRI". Magnetic Resonance Imaging. 109: 165–172. doi:10.1016/j.mri.2024.03.027. PMID 38513785.
- ↑ Huettel SA, Song AW, McCarthy G (2009). Functional Magnetic Resonance Imaging (2nd ed.). Sunderland, Massachusetts: Sinauer Associates. p. 26. ISBN 978-0-87893-286-3.
- ↑ Detre JA, Rao H, Wang DJ, Chen YF, Wang Z (May 2012). "Applications of arterial spin labeled MRI in the brain". Journal of Magnetic Resonance Imaging. 35 (5): 1026–37. doi:10.1002/jmri.23581. PMC 3326188 . PMID 22246782.
- ↑ Roberts, D A; Detre, J A; Bolinger, L; Insko, E K; Lenkinski, R E; Pentecost, M J; Leigh, J S (1995-07-01). "Renal perfusion in humans: MR imaging with spin tagging of arterial water". Radiology. 196 (1): 281–286. doi:10.1148/radiology.196.1.7784582. ISSN 0033-8419. PMID 7784582.
- ↑ Taso, Manuel; Guidon, Arnaud; Zhao, Li; Mortele, Koenraad J.; Alsop, David C. (2019). "Pancreatic perfusion and arterial-transit-time quantification using pseudocontinuous arterial spin labeling at 3T". Magnetic Resonance in Medicine. 81 (1): 542–550. doi: 10.1002/mrm.27435 . ISSN 1522-2594. PMID 30229559.
- ↑ Watson RE (2015-10-01). "Lessons Learned from MRI Safety Events". Current Radiology Reports. 3 (10): 37. doi:10.1007/s40134-015-0122-z. ISSN 2167-4825. S2CID 57880401.