In mammals, pregnancy is the period of reproduction during which a female carries one or more live offspring from implantation in the uterus through gestation. It begins when a fertilized zygote implants in the female's uterus, and ends once it leaves the uterus.

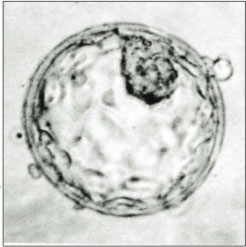
An embryo is an initial stage of development of a multicellular organism. In organisms that reproduce sexually, embryonic development is the part of the life cycle that begins just after fertilization of the female egg cell by the male sperm cell. The resulting fusion of these two cells produces a single-celled zygote that undergoes many cell divisions that produce cells known as blastomeres. The blastomeres are arranged as a solid ball that when reaching a certain size, called a morula, takes in fluid to create a cavity called a blastocoel. The structure is then termed a blastula, or a blastocyst in mammals.
The amniotic sac, also called the bag of waters or the membranes, is the sac in which the embryo and later fetus develops in amniotes. It is a thin but tough transparent pair of membranes that hold a developing embryo until shortly before birth. The inner of these membranes, the amnion, encloses the amniotic cavity, containing the amniotic fluid and the embryo. The outer membrane, the chorion, contains the amnion and is part of the placenta. On the outer side, the amniotic sac is connected to the yolk sac, the allantois, and via the umbilical cord, the placenta.
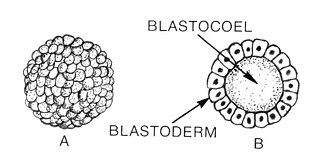
Blastulation is the stage in early animal embryonic development that produces the blastula. In mammalian development the blastula develops into the blastocyst with a differentiated inner cell mass and an outer trophectoderm. The blastula is a hollow sphere of cells known as blastomeres surrounding an inner fluid-filled cavity called the blastocoel. Embryonic development begins with a sperm fertilizing an egg cell to become a zygote, which undergoes many cleavages to develop into a ball of cells called a morula. Only when the blastocoel is formed does the early embryo become a blastula. The blastula precedes the formation of the gastrula in which the germ layers of the embryo form.

The blastocoel, also spelled blastocoele and blastocele, and also called cleavage cavity, or segmentation cavity is a fluid-filled or yolk-filled cavity that forms in the blastula during very early embryonic development. At this stage in mammals the blastula develops into the blastocyst containing an inner cell mass, and outer trophectoderm.

The trophoblast is the outer layer of cells of the blastocyst. Trophoblasts are present four days after fertilization in humans. They provide nutrients to the embryo and develop into a large part of the placenta. They form during the first stage of pregnancy and are the first cells to differentiate from the fertilized egg to become extraembryonic structures that do not directly contribute to the embryo. After blastulation, the trophoblast is contiguous with the ectoderm of the embryo and is referred to as the trophectoderm. After the first differentiation, the cells in the human embryo lose their totipotency because they can no longer form a trophoblast. They become pluripotent stem cells.
A germ layer is a primary layer of cells that forms during embryonic development. The three germ layers in vertebrates are particularly pronounced; however, all eumetazoans produce two or three primary germ layers. Some animals, like cnidarians, produce two germ layers making them diploblastic. Other animals such as bilaterians produce a third layer between these two layers, making them triploblastic. Germ layers eventually give rise to all of an animal's tissues and organs through the process of organogenesis.

In developmental biology, animal embryonic development, also known as animal embryogenesis, is the developmental stage of an animal embryo. Embryonic development starts with the fertilization of an egg cell (ovum) by a sperm cell, (spermatozoon). Once fertilized, the ovum becomes a single diploid cell known as a zygote. The zygote undergoes mitotic divisions with no significant growth and cellular differentiation, leading to development of a multicellular embryo after passing through an organizational checkpoint during mid-embryogenesis. In mammals, the term refers chiefly to the early stages of prenatal development, whereas the terms fetus and fetal development describe later stages.

"Cytotrophoblast" is the name given to both the inner layer of the trophoblast or the cells that live there. It is interior to the syncytiotrophoblast and external to the wall of the blastocyst in a developing embryo.
In embryology, Carnegie stages are a standardized system of 23 stages used to provide a unified developmental chronology of the vertebrate embryo.

The inner cell mass (ICM) or embryoblast is a structure in the early development of an embryo. It is the mass of cells inside the blastocyst that will eventually give rise to the definitive structures of the fetus. The inner cell mass forms in the earliest stages of embryonic development, before implantation into the endometrium of the uterus. The ICM is entirely surrounded by the single layer of trophoblast cells of the trophectoderm.

In amniote embryonic development, the epiblast is one of two distinct cell layers arising from the inner cell mass in the mammalian blastocyst, or from the blastula in reptiles and birds, the other layer is the hypoblast. It drives the embryo proper through its differentiation into the three primary germ layers, ectoderm, mesoderm and endoderm, during gastrulation. The amniotic ectoderm and extraembryonic mesoderm also originate from the epiblast.

Implantation, also known as nidation, is the stage in the embryonic development of mammals in which the blastocyst hatches, attaches, adheres, and invades into the wall of the female's uterus. Implantation is the first stage of gestation, and, when successful, the female is considered to be pregnant. An implanted embryo is detected by the presence of increased levels of human chorionic gonadotropin (hCG) in a pregnancy test. The implanted embryo will receive oxygen and nutrients in order to grow.

The bilaminar embryonic disc, bilaminar blastoderm or embryonic disc is the distinct two-layered structure of cells formed in an embryo. In the development of the human embryo this takes place by day eight. It is formed when the inner cell mass, also known as the embryoblast, forms a bilaminar disc of two layers, an upper layer called the epiblast and a lower layer called the hypoblast, which will eventually form into fetus. These two layers of cells are stretched between two fluid-filled cavities at either end: the primitive yolk sac and the amniotic sac.

Human embryonic development or human embryogenesis is the development and formation of the human embryo. It is characterised by the processes of cell division and cellular differentiation of the embryo that occurs during the early stages of development. In biological terms, the development of the human body entails growth from a one-celled zygote to an adult human being. Fertilization occurs when the sperm cell successfully enters and fuses with an egg cell (ovum). The genetic material of the sperm and egg then combine to form the single cell zygote and the germinal stage of development commences. Embryonic development in the human, covers the first eight weeks of development; at the beginning of the ninth week the embryo is termed a fetus. The eight weeks has 23 stages.

In amniote embryology, the hypoblast is one of two distinct layers arising from the inner cell mass in the mammalian blastocyst, or from the blastodisc in reptiles and birds. The hypoblast gives rise to the yolk sac, which in turn gives rise to the chorion.
Morphokinetics (‘morpho’’ form/shape and ‘kinetics’ movement) refers to time specific morphological changes during embryo development providing dynamic information on a fertilized egg. The detailed information eases morphological selection of embryos with high implantation potential to be used in In-Vitro Fertilisation treatment.

Preimplantation factor(PIF) is a peptide secreted by trophoblast cells prior to placenta formation in early embryonic development. Human embryos begin to express PIF at the 4-cell stage, with expression increasing by the morula stage and continuing to do so throughout the first trimester. Expression of preimplantation factor in the blastocyst was discovered as an early correlate of the viability of the eventual pregnancy. Preimplantation factor was identified in 1994 by a lymphocyte platelet-binding assay, where it was thought to be an early biomarker of pregnancy. It has a simple primary structure with a short sequence of fifteen amino acids without any known quaternary structure. A synthetic analogue of preimplantation factor (commonly abbreviated in studies as sPIF or PIF*) that has an identical amino acid sequence and mimics the normal biological activity of PIF has been developed and is commonly used in research studies, particularly those that aim to study potential adult therapeutics.
Reichert's membrane is an extraembryonic membrane that forms during early mammalian embryonic development. It forms as a thickened basement membrane to cover the embryo immediately following implantation to give protection to the embryo from the uterine pressures exerted. Reichert's membrane is also important for the maternofetal exchange of nutrients. The membrane collapses once the placenta has fully developed.
This glossary of developmental biology is a list of definitions of terms and concepts commonly used in the study of developmental biology and related disciplines in biology, including embryology and reproductive biology, primarily as they pertain to vertebrate animals and particularly to humans and other mammals. The developmental biology of invertebrates, plants, fungi, and other organisms is treated in other articles; e.g terms relating to the reproduction and development of insects are listed in Glossary of entomology, and those relating to plants are listed in Glossary of botany.