Pattern recognition receptors (PRRs) play a crucial role in the proper function of the innate immune system. PRRs are germline-encoded host sensors, which detect molecules typical for the pathogens. They are proteins expressed mainly by cells of the innate immune system, such as dendritic cells, macrophages, monocytes, neutrophils, as well as by epithelial cells, to identify two classes of molecules: pathogen-associated molecular patterns (PAMPs), which are associated with microbial pathogens, and damage-associated molecular patterns (DAMPs), which are associated with components of host's cells that are released during cell damage or death. They are also called primitive pattern recognition receptors because they evolved before other parts of the immune system, particularly before adaptive immunity. PRRs also mediate the initiation of antigen-specific adaptive immune response and release of inflammatory cytokines.
Scavenger receptors are a large and diverse superfamily of cell surface receptors. Its properties were first recorded in 1970 by Drs. Brown and Goldstein, with the defining property being the ability to bind and remove modified low density lipoproteins (LDL). Today scavenger receptors are known to be involved in a wide range of processes, such as: homeostasis, apoptosis, inflammatory diseases and pathogen clearance. Scavenger receptors are mainly found on myeloid cells and other cells that bind to numerous ligands, primarily endogenous and modified host-molecules together with pathogen-associated molecular patterns(PAMPs), and remove them. The Kupffer cells in the liver are particularly rich in scavenger receptors, includes SR-A I, SR-A II, and MARCO.
Collectins (collagen-containing C-type lectins) are a part of the innate immune system. They form a family of collagenous Ca2+-dependent defense lectins, which are found in animals. Collectins are soluble pattern recognition receptors (PRRs). Their function is to bind to oligosaccharide structure or lipids that are on the surface of microorganisms. Like other PRRs they bind pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs) of oligosaccharide origin. Binding of collectins to microorganisms may trigger elimination of microorganisms by aggregation, complement activation, opsonization, activation of phagocytosis, or inhibition of microbial growth. Other functions of collectins are modulation of inflammatory, allergic responses, adaptive immune system and clearance of apoptotic cells.
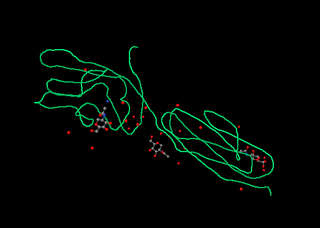
CD2 is a cell adhesion molecule found on the surface of T cells and natural killer (NK) cells. It has also been called T-cell surface antigen T11/Leu-5, LFA-2, LFA-3 receptor, erythrocyte receptor and rosette receptor.
Siglecs(Sialic acid-binding immunoglobulin-type lectins) are cell surface proteins that bind sialic acid. They are found primarily on the surface of immune cells and are a subset of the I-type lectins. There are 14 different mammalian Siglecs, providing an array of different functions based on cell surface receptor-ligand interactions.

CD94, also known as killer cell lectin-like receptor subfamily D, member 1 (KLRD1) is a human gene.

C-type lectin domain family 4 member M is a protein that in humans is encoded by the CLEC4M gene. CLEC4M has also been designated as CD299.

C-type lectin domain family 7 member A or Dectin-1 is a protein that in humans is encoded by the CLEC7A gene. CLEC7A is a member of the C-type lectin/C-type lectin-like domain (CTL/CTLD) superfamily. The encoded glycoprotein is a small type II membrane receptor with an extracellular C-type lectin-like domain fold and a cytoplasmic domain with a partial immunoreceptor tyrosine-based activation motif. It functions as a pattern-recognition receptor for a variety of β-1,3-linked and β-1,6-linked glucans from fungi and plants, and in this way plays a role in innate immune response. Expression is found on myeloid dendritic cells, monocytes, macrophages and B cells. Alternate transcriptional splice variants, encoding different isoforms, have been characterized. This gene is closely linked to other CTL/CTLD superfamily members on chromosome 12p13 in the natural killer gene complex region.

NKG2-F type II integral membrane protein is a protein that in humans is encoded by the KLRC4 gene.

Sialic acid-binding Ig-like lectin 7 is a protein that in humans is encoded by the SIGLEC7 gene. SIGLEC7 has also been designated as CD328.

NKG2-C type II integral membrane protein or NKG2C is a protein that in humans is encoded by the KLRC2 gene. It is also known as or cluster of differentiation 159c (CD159c).

C-type lectin domain family 1 member B is a protein that in humans is encoded by the CLEC1B gene.

Endosialin is a protein that in humans is encoded by the CD248 gene.

C-type lectin domain family 4 member A is a protein that in humans is encoded by the CLEC4A gene.

C-type lectin domain family 1 member A is a protein that in humans is encoded by the CLEC1A gene.

C-type lectin domain family 2 member B is a protein that in humans is encoded by the CLEC2B gene.

C-type lectin domain family 12 member A is a protein that in humans is encoded by the CLEC12A gene.

The nucleotide-binding oligomerization domain-like receptors, or NOD-like receptors (NLRs), are intracellular sensors of pathogen-associated molecular patterns (PAMPs) that enter the cell via phagocytosis or pores, and damage-associated molecular patterns (DAMPs) that are associated with cell stress. They are types of pattern recognition receptors (PRRs), and play key roles in the regulation of innate immune response. NLRs can cooperate with toll-like receptors (TLRs) and regulate inflammatory and apoptotic response.
Glycan arrays, like that offered by the Consortium for Functional Glycomics (CFG), National Center for Functional Glycomics (NCFG) and Z Biotech, LLC, contain carbohydrate compounds that can be screened with lectins, antibodies or cell receptors to define carbohydrate specificity and identify ligands. Glycan array screening works in much the same way as other microarray that is used for instance to study gene expression DNA microarrays or protein interaction Protein microarrays.

Collectin-12, also known as collectin subfamily member 12, is a collectin protein that in humans is encoded by the COLEC12 gene.









