Cell adhesion is the process by which cells interact and attach to neighbouring cells through specialised molecules of the cell surface. This process can occur either through direct contact between cell surfaces such as cell junctions or indirect interaction, where cells attach to surrounding extracellular matrix, a gel-like structure containing molecules released by cells into spaces between them. Cells adhesion occurs from the interactions between cell-adhesion molecules (CAMs), transmembrane proteins located on the cell surface. Cell adhesion links cells in different ways and can be involved in signal transduction for cells to detect and respond to changes in the surroundings. Other cellular processes regulated by cell adhesion include cell migration and tissue development in multicellular organisms. Alterations in cell adhesion can disrupt important cellular processes and lead to a variety of diseases, including cancer and arthritis. Cell adhesion is also essential for infectious organisms, such as bacteria or viruses, to cause diseases.
Cell adhesion molecules (CAMs) are a subset of cell surface proteins that are involved in the binding of cells with other cells or with the extracellular matrix (ECM), in a process called cell adhesion. In essence, CAMs help cells stick to each other and to their surroundings. CAMs are crucial components in maintaining tissue structure and function. In fully developed animals, these molecules play an integral role in generating force and movement and consequently ensuring that organs are able to execute their functions normally. In addition to serving as "molecular glue", CAMs play important roles in the cellular mechanisms of growth, contact inhibition, and apoptosis. Aberrant expression of CAMs may result in a wide range of pathologies, ranging from frostbite to cancer.

Selectin P ligand, also known as SELPLG or CD162, is a human gene.

The CD44 antigen is a cell-surface glycoprotein involved in cell–cell interactions, cell adhesion and migration. In humans, the CD44 antigen is encoded by the CD44 gene on chromosome 11. CD44 has been referred to as HCAM, Pgp-1, Hermes antigen, lymphocyte homing receptor, ECM-III, and HUTCH-1.

P-selectin is a type-1 transmembrane protein that in humans is encoded by the SELP gene.

Versican is a large extracellular matrix proteoglycan that is present in a variety of human tissues. It is encoded by the VCAN gene.

ICAM-1 also known as CD54 is a protein that in humans is encoded by the ICAM1 gene. This gene encodes a cell surface glycoprotein which is typically expressed on endothelial cells and cells of the immune system. It binds to integrins of type CD11a / CD18, or CD11b / CD18 and is also exploited by rhinovirus as a receptor for entry into respiratory epithelium.
L-selectin, also known as CD62L, is a cell adhesion molecule found on the cell surface of leukocytes, and the blastocyst. It is coded for in the human by the SELL gene. L-selectin belongs to the selectin family of proteins, which recognize sialylated carbohydrate groups containing a Sialyl LewisX (sLeX) determinant. L-selectin plays an important role in both the innate and adaptive immune responses by facilitating leukocyte-endothelial cell adhesion events. These tethering interactions are essential for the trafficking of monocytes and neutrophils into inflamed tissue as well as the homing of lymphocytes to secondary lymphoid organs. L-selectin is also expressed by lymphoid primed hematopoietic stem cells and may participate in the migration of these stem cells to the primary lymphoid organs. In addition to its function in the immune response, L-selectin is expressed on embryonic cells and facilitates the attachment of the blastocyst to the endometrial endothelium during human embryo implantation.

E-selectin, also known as CD62 antigen-like family member E (CD62E), endothelial-leukocyte adhesion molecule 1 (ELAM-1), or leukocyte-endothelial cell adhesion molecule 2 (LECAM2), is a selectin cell adhesion molecule expressed only on endothelial cells activated by cytokines. Like other selectins, it plays an important part in inflammation. In humans, E-selectin is encoded by the SELE gene.
High endothelial venules (HEV) are specialized post-capillary venules characterized by plump endothelial cells as opposed to the usual flatter endothelial cells found in regular venules. HEVs enable lymphocytes circulating in the blood to directly enter a lymph node.
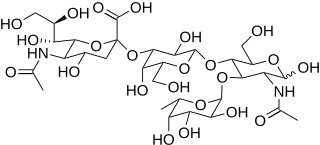
Sialyl LewisX (sLeX), also known as cluster of differentiation 15s (CD15s) or stage-specific embryonic antigen 1 (SSEA-1), is a tetrasaccharide carbohydrate which is usually attached to O-glycans on the surface of cells. It is known to play a vital role in cell-to-cell recognition processes. It is also the means by which an egg attracts sperm; first, to stick to it, then bond with it and eventually form a zygote.

Galectins are a class of proteins that bind specifically to β-galactoside sugars, such as N-acetyllactosamine, which can be bound to proteins by either N-linked or O-linked glycosylation. They are also termed S-type lectins due to their dependency on disulphide bonds for stability and carbohydrate binding. There have been about 15 galectins discovered in mammals, encoded by the LGALS genes, which are numbered in a consecutive manner. Only galectin-1, -2, -3, -4, -7, -7B, -8, -9, -9B, 9C, -10, -12, -13, -14, and -16 have been identified in humans. Galectin-5 and -6 are found in rodents, whereas galectin-11 and -15 are uniquely found in sheep and goats. Members of the galectin family have also been discovered in other mammals, birds, amphibians, fish, nematodes, sponges, and some fungi. Unlike the majority of lectins they are not membrane bound, but soluble proteins with both intra- and extracellular functions. They have distinct but overlapping distributions but found primarily in the cytosol, nucleus, extracellular matrix or in circulation. Although many galectins must be secreted, they do not have a typical signal peptide required for classical secretion. The mechanism and reason for this non-classical secretion pathway is unknown.
The mannose receptor is a C-type lectin primarily present on the surface of macrophages, immature dendritic cells and liver sinusoidal endothelial cells, but is also expressed on the surface of skin cells such as human dermal fibroblasts and keratinocytes. It is the first member of a family of endocytic receptors that includes Endo180 (CD280), M-type PLA2R, and DEC-205 (CD205).
Glycosylation-dependent cell adhesion molecule-1 (GLYCAM1) is a proteoglycan ligand expressed on cells of the high endothelial venules in lymphoid tissues. It is the ligand for the receptor L-selectin allowing for naive lymphocytes to exit the bloodstream into lymphoid tissues. GLYCAM1 binds to L-selectin by presenting one or more O-linked carbohydrates to the lectin domain of the leukocyte cell surface selectin. Data suggests that GLYCAM1 is a hormone-regulated milk protein that is part of the milk mucin complex.

In molecular biology, CD18 is an integrin beta chain protein that is encoded by the ITGB2 gene in humans. Upon binding with one of a number of alpha chains, CD18 is capable of forming multiple heterodimers, which play significant roles in cellular adhesion and cell surface signaling, as well as important roles in immune responses. CD18 also exists in soluble, ligand binding forms. Deficiencies in CD18 expression can lead to adhesion defects in circulating white blood cells in humans, reducing the immune system's ability to fight off foreign invaders.

Leukocyte extravasation is the movement of leukocytes out of the circulatory system and towards the site of tissue damage or infection. This process forms part of the innate immune response, involving the recruitment of non-specific leukocytes. Monocytes also use this process in the absence of infection or tissue damage during their development into macrophages.

Alpha-(1,3)-fucosyltransferase is an enzyme that in humans is encoded by the FUT7 gene.

Sialic acid-binding Ig-like lectin 8 is a protein that in humans is encoded by the SIGLEC8 gene. This gene is located on chromosome 19q13.4, about 330 kb downstream of the SIGLEC9 gene. Within the siglec family of transmembrane proteins, Siglec-8 belongs to the CD33-related siglec subfamily, a subfamily that has undergone rapid evolution.
A catch bond is a type of noncovalent bond whose dissociation lifetime increases with tensile force applied to the bond. Normally, bond lifetimes are expected to diminish with force. In the case of catch bonds, the lifetime of the bond actually increases up to a maximum before it decreases like in a normal bond. Catch bonds work in a way that is conceptually similar to that of a Chinese finger trap. While catch bonds are strengthened by an increase in force, the force increase is not necessary for the bond to work. Catch bonds were suspected for many years to play a role in the rolling of leukocytes, being strong enough to roll in presence of high forces caused by high shear stresses, while avoiding getting stuck in capillaries where the fluid flow, and therefore shear stress, is low. The existence of catch bonds was debated for many years until strong evidence of their existence was found in bacteria. Definite proof of their existence came shortly thereafter in leukocytes.

Endothelial cell anergy is a condition during the process of angiogenesis, where endothelial cells, the cells that line the inside of blood vessels, can no longer respond to inflammatory cytokines. These cytokines are necessary to induce the expression of cell adhesion molecules to allow leukocyte infiltration from the blood into the tissue at places of inflammation, such as a tumor. This condition, which protects the tumor from the immune system, is the result of exposure to angiogenic growth factors.