Cell adhesion is the process by which cells interact and attach to neighbouring cells through specialised molecules of the cell surface. This process can occur either through direct contact between cell surfaces such as cell junctions or indirect interaction, where cells attach to surrounding extracellular matrix, a gel-like structure containing molecules released by cells into spaces between them. Cells adhesion occurs from the interactions between cell-adhesion molecules (CAMs), transmembrane proteins located on the cell surface. Cell adhesion links cells in different ways and can be involved in signal transduction for cells to detect and respond to changes in the surroundings. Other cellular processes regulated by cell adhesion include cell migration and tissue development in multicellular organisms. Alterations in cell adhesion can disrupt important cellular processes and lead to a variety of diseases, including cancer and arthritis. Cell adhesion is also essential for infectious organisms, such as bacteria or viruses, to cause diseases.

L1, also known as L1CAM, is a transmembrane protein member of the L1 protein family, encoded by the L1CAM gene. This protein, of 200-220 kDa, is a neuronal cell adhesion molecule with a strong implication in cell migration, adhesion, neurite outgrowth, myelination and neuronal differentiation. It also plays a key role in treatment-resistant cancers due to its function. It was first identified in 1984 by M. Schachner who found the protein in post-mitotic mice neurons.

The selectins are a family of cell adhesion molecules. All selectins are single-chain transmembrane glycoproteins that share similar properties to C-type lectins due to a related amino terminus and calcium-dependent binding. Selectins bind to sugar moieties and so are considered to be a type of lectin, cell adhesion proteins that bind sugar polymers.

P-selectin is a type-1 transmembrane protein that in humans is encoded by the SELP gene.

ICAM-1 also known as CD54 is a protein that in humans is encoded by the ICAM1 gene. This gene encodes a cell surface glycoprotein which is typically expressed on endothelial cells and cells of the immune system. It binds to integrins of type CD11a / CD18, or CD11b / CD18 and is also exploited by rhinovirus as a receptor for entry into respiratory epithelium.

E-selectin, also known as CD62 antigen-like family member E (CD62E), endothelial-leukocyte adhesion molecule 1 (ELAM-1), or leukocyte-endothelial cell adhesion molecule 2 (LECAM2), is a selectin cell adhesion molecule expressed only on endothelial cells activated by cytokines. Like other selectins, it plays an important part in inflammation. In humans, E-selectin is encoded by the SELE gene.

Vascular cell adhesion protein 1 also known as vascular cell adhesion molecule 1 (VCAM-1) or cluster of differentiation 106 (CD106) is a protein that in humans is encoded by the VCAM1 gene. VCAM-1 functions as a cell adhesion molecule.

Tyrosine-protein phosphatase non-receptor type 11 (PTPN11) also known as protein-tyrosine phosphatase 1D (PTP-1D), Src homology region 2 domain-containing phosphatase-2 (SHP-2), or protein-tyrosine phosphatase 2C (PTP-2C) is an enzyme that in humans is encoded by the PTPN11 gene. PTPN11 is a protein tyrosine phosphatase (PTP) Shp2.

CD146 also known as the melanoma cell adhesion molecule (MCAM) or cell surface glycoprotein MUC18, is a 113kDa cell adhesion molecule currently used as a marker for endothelial cell lineage. In humans, the CD146 protein is encoded by the MCAM gene.

Leukocyte extravasation is the movement of leukocytes out of the circulatory system and towards the site of tissue damage or infection. This process forms part of the innate immune response, involving the recruitment of non-specific leukocytes. Monocytes also use this process in the absence of infection or tissue damage during their development into macrophages.
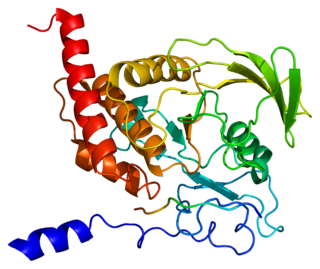
Tyrosine-protein phosphatase non-receptor type 6, also known as Src homology region 2 domain-containing phosphatase-1 (SHP-1), is an enzyme that in humans is encoded by the PTPN6 gene.
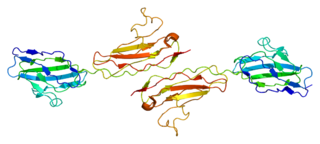
Junctional adhesion molecule A is a protein that in humans is encoded by the F11R gene. It has also been designated as CD321.

CD93 is a protein that in humans is encoded by the CD93 gene. CD93 is a C-type lectin transmembrane receptor which plays a role not only in cell–cell adhesion processes but also in host defense.

Junctional adhesion molecule C is a protein that in humans is encoded by the JAM3 gene.
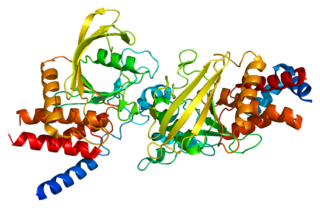
Receptor-type tyrosine-protein phosphatase beta or VE-PTP is an enzyme specifically expressed in endothelial cells that in humans is encoded by the PTPRB gene.

Receptor-type tyrosine-protein phosphatase mu is an enzyme that in humans is encoded by the PTPRM gene.

CD226, PTA1 or DNAM-1 is a ~65 kDa immunoglobulin-like transmembrane glycoprotein expressed on the surface of natural killer cells, NK T cell, B cells, dendritic cells, hematopoietic precursor cells, platelets, monocytes and T cells.

Tyrosine kinase with immunoglobulin-like and EGF-like domains 1 also known as TIE1 is an angiopoietin receptor which in humans is encoded by the TIE1 gene.

Fermitin family homolog 3) (FERMT3), also known as kindlin-3 (KIND3), MIG2-like protein (MIG2B), or unc-112-related protein 2 (URP2) is a protein that in humans is encoded by the FERMT3 gene. The kindlin family of proteins, member of the B4.1 superfamily, comprises three conserved protein homologues, kindlin 1, 2, and 3. They each contain a bipartite FERM domain comprising four subdomains F0, F1, F2, and F3 that show homology with the FERM head (H) domain of the cytoskeletal Talin protein. Kindlins have been linked to Kindler syndrome, leukocyte adhesion deficiency, cancer and other acquired human diseases. They are essential in the organisation of focal adhesions that mediate cell-extracellular matrix junctions and are involved in other cellular compartments that control cell-cell contacts and nucleus functioning. Therefore, they are responsible for cell to cell crosstalk via cell-cell contacts and integrin mediated cell adhesion through focal adhesion proteins and as specialised adhesion structures of hematopoietic cells they are also present in podosome's F actin surrounding ring structure. Isoform 2 may act as a repressor of NF-kappa-B and apoptosis

Endothelial cell anergy is a condition during the process of angiogenesis, where endothelial cells, the cells that line the inside of blood vessels, can no longer respond to inflammatory cytokines. These cytokines are necessary to induce the expression of cell adhesion molecules to allow leukocyte infiltration from the blood into the tissue at places of inflammation, such as a tumor. This condition, which protects the tumor from the immune system, is the result of exposure to angiogenic growth factors.