Nicotinamide adenine dinucleotide (NAD) is a coenzyme central to metabolism. Found in all living cells, NAD is called a dinucleotide because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine nucleobase and the other, nicotinamide. NAD exists in two forms: an oxidized and reduced form, abbreviated as NAD+ and NADH (H for hydrogen), respectively.

Poly (ADP-ribose) polymerase (PARP) is a family of proteins involved in a number of cellular processes such as DNA repair, genomic stability, and programmed cell death.

Cyclic ADP-ribose, frequently abbreviated as cADPR, is a cyclic adenine nucleotide (like cAMP) with two phosphate groups present on 5' OH of the adenosine (like ADP), further connected to another ribose at the 5' position, which, in turn, closes the cycle by glycosidic bonding to the nitrogen 1 (N1) of the same adenine base (whose position N9 has the glycosidic bond to the other ribose). The N1-glycosidic bond to adenine is what distinguishes cADPR from ADP-ribose (ADPR), the non-cyclic analog. cADPR is produced from nicotinamide adenine dinucleotide (NAD+) by ADP-ribosyl cyclases (EC 3.2.2.5) as part of a second messenger system.

Nicotinic acid adenine dinucleotide phosphate (NAADP) is a Ca2+-mobilizing second messenger synthesised in response to extracellular stimuli. Like its mechanistic cousins, IP3 and cyclic adenosine diphosphoribose (Cyclic ADP-ribose), NAADP binds to and opens Ca2+ channels on intracellular organelles, thereby increasing the intracellular Ca2+ concentration which, in turn, modulates sundry cellular processes (see Calcium signalling). Structurally, it is a dinucleotide that only differs from the house-keeping enzyme cofactor, NADP by a hydroxyl group (replacing the nicotinamide amino group) and yet this minor modification converts it into the most potent Ca2+-mobilizing second messenger yet described. NAADP acts across phyla from plants to humans.

Adenosine diphosphate ribose (ADPR) is an ester molecule formed into chains by the enzyme poly ADP ribose polymerase. ADPR is created from cyclic ADP-ribose (cADPR) by the CD38 enzyme using nicotinamide adenine dinucleotide (NAD+) as a cofactor.

Histone-modifying enzymes are enzymes involved in the modification of histone substrates after protein translation and affect cellular processes including gene expression. To safely store the eukaryotic genome, DNA is wrapped around four core histone proteins, which then join to form nucleosomes. These nucleosomes further fold together into highly condensed chromatin, which renders the organism's genetic material far less accessible to the factors required for gene transcription, DNA replication, recombination and repair. Subsequently, eukaryotic organisms have developed intricate mechanisms to overcome this repressive barrier imposed by the chromatin through histone modification, a type of post-translational modification which typically involves covalently attaching certain groups to histone residues. Once added to the histone, these groups elicit either a loose and open histone conformation, euchromatin, or a tight and closed histone conformation, heterochromatin. Euchromatin marks active transcription and gene expression, as the light packing of histones in this way allows entry for proteins involved in the transcription process. As such, the tightly packed heterochromatin marks the absence of current gene expression.

ADP-ribosylation is the addition of one or more ADP-ribose moieties to a protein. It is a reversible post-translational modification that is involved in many cellular processes, including cell signaling, DNA repair, gene regulation and apoptosis. Improper ADP-ribosylation has been implicated in some forms of cancer. It is also the basis for the toxicity of bacterial compounds such as cholera toxin, diphtheria toxin, and others.

Poly [ADP-ribose] polymerase 1 (PARP-1) also known as NAD+ ADP-ribosyltransferase 1 or poly[ADP-ribose] synthase 1 is an enzyme that in humans is encoded by the PARP1 gene. It is the most abundant of the PARP family of enzymes, accounting for 90% of the NAD+ used by the family. PARP1 is mostly present in cell nucleus, but cytosolic fraction of this protein was also reported.

ADP-ribose diphosphatase (EC 3.6.1.13) is an enzyme that catalyzes a hydrolysis reaction in which water nucleophilically attacks ADP-ribose to produce AMP and D-ribose 5-phosphate. Enzyme hydrolysis occurs by the breakage of a phosphoanhydride bond and is dependent on Mg2+ ions that are held in complex by the enzyme.
In enzymology, a NAD+ glycohydrolase (EC 3.2.2.5) is an enzyme that catalyzes the chemical reaction

In enzymology, a ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase (EC 3.2.2.6) is a bifunctional enzyme that catalyzes the chemical reaction

Poly [ADP-ribose] polymerase 4 is an enzyme that in humans is encoded by the PARP4 gene.

Bst1 is an enzyme that in humans is encoded by the BST1 gene. CD157 is a paralog of CD38, both of which are located on chromosome 4 (4p15) in humans.
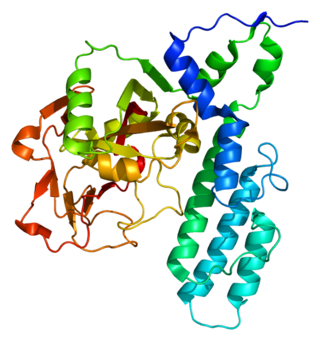
Poly [ADP-ribose] polymerase 3 is an enzyme that in humans is encoded by the PARP3 gene.

Poly [ADP-ribose] polymerase 2 is an enzyme that in humans is encoded by the PARP2 gene. It is one of the PARP family of enzymes.
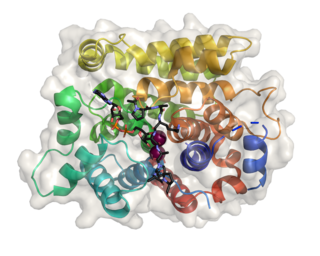
(ADP-ribosyl)hydrolase 3 (ARH3) is an enzyme that in humans is encoded by the ADPRHL2 gene (also called ADPRS). This enzyme reverses the proteins’ post-translational addition of ADP-ribose to serine residues as part of the DNA damage response The enzyme is also known to cleave poly(ADP-ribose) polymers, 1''-O-acetyl-ADP-ribose and alpha-NAD+

In molecular biology, the (ADP-ribosyl)hydrolase (ARH) family contains enzymes which catalyses the hydrolysis of ADP-ribosyl modifications from proteins, nucleic acids and small molecules.

Daratumumab, sold under the brand name Darzalex, is an anti-cancer monoclonal antibody medication. It binds to CD38, which is overexpressed in multiple myeloma cells. Daratumumab was originally developed by Genmab, but it is now being jointly developed by Genmab along with the Johnson & Johnson subsidiary Janssen Biotech, which acquired worldwide commercialization rights to the drug from Genmab.

Isatuximab, sold under the brand name Sarclisa, is a monoclonal antibody (mAb) medication for the treatment of multiple myeloma.

Sterile alpha and TIR motif containing 1 Is an enzyme that in humans is encoded by the SARM1 gene. It is the most evolutionarily conserved member of the Toll/Interleukin receptor-1 (TIR) family. SARM1's TIR domain has intrinsic NADase enzymatic activity that is highly conserved from archaea, plants, nematode worms, fruit flies, and humans. In mammals, SARM1 is highly expressed in neurons, where it resides in both cell bodies and axons, and can be associated with mitochondria.