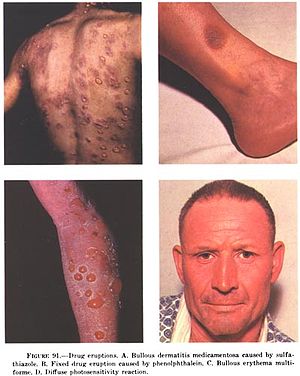
Stevens–Johnson syndrome (SJS) is a type of severe skin reaction. Together with toxic epidermal necrolysis (TEN) and Stevens–Johnson/toxic epidermal necrolysis (SJS/TEN), it forms a spectrum of disease, with SJS being less severe. Erythema multiforme (EM) is generally considered a separate condition. Early symptoms of SJS include fever and flu-like symptoms. A few days later, the skin begins to blister and peel, forming painful raw areas. Mucous membranes, such as the mouth, are also typically involved. Complications include dehydration, sepsis, pneumonia and multiple organ failure.

Allopurinol is a medication used to decrease high blood uric acid levels. It is specifically used to prevent gout, prevent specific types of kidney stones and for the high uric acid levels that can occur with chemotherapy. It is taken by mouth or injected into a vein.

Sulfonamide is a functional group that is the basis of several groups of drugs, which are called sulphonamides, sulfa drugs or sulpha drugs. The original antibacterial sulfonamides are synthetic (nonantibiotic) antimicrobial agents that contain the sulfonamide group. Some sulfonamides are also devoid of antibacterial activity, e.g., the anticonvulsant sultiame. The sulfonylureas and thiazide diuretics are newer drug groups based upon the antibacterial sulfonamides.
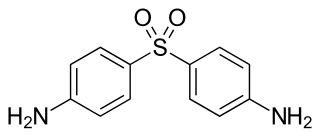
Dapsone, also known as 4,4'-sulfonyldianiline (SDA) or diaminodiphenyl sulfone (DDS), is an antibiotic commonly used in combination with rifampicin and clofazimine for the treatment of leprosy. It is a second-line medication for the treatment and prevention of pneumocystis pneumonia and for the prevention of toxoplasmosis in those who have poor immune function. Additionally, it has been used for acne, dermatitis herpetiformis, and various other skin conditions. Dapsone is available both topically and by mouth.

Abacavir, sold under the brand name Ziagen among others, is a medication used to treat HIV/AIDS. Similar to other nucleoside analog reverse-transcriptase inhibitors (NRTIs), abacavir is used together with other HIV medications, and is not recommended by itself. It is taken by mouth as a tablet or solution and may be used in children over the age of three months.

Toxic epidermal necrolysis (TEN) is a type of severe skin reaction. Together with Stevens–Johnson syndrome (SJS) it forms a spectrum of disease, with TEN being more severe. Early symptoms include fever and flu-like symptoms. A few days later the skin begins to blister and peel forming painful raw areas. Mucous membranes, such as the mouth, are also typically involved. Complications include dehydration, sepsis, pneumonia, and multiple organ failure.

Sulfamethoxazole is an antibiotic. It is used for bacterial infections such as urinary tract infections, bronchitis, and prostatitis and is effective against both gram negative and positive bacteria such as Listeria monocytogenes and E. coli.

Tetrazepam is a benzodiazepine derivative with anticonvulsant, anxiolytic, muscle relaxant and slightly hypnotic properties. It was formerly used mainly in Austria, France, Belgium, Germany and Spain to treat muscle spasm, anxiety disorders such as panic attacks, or more rarely to treat depression, premenstrual syndrome or agoraphobia. Tetrazepam has relatively little sedative effect at low doses while still producing useful muscle relaxation and anxiety relief. The Co-ordination Group for Mutual Recognition and Decentralised Procedures-Human endorsed the Pharmacovigilance Risk Assessment Committee (PRAC) recommendation to suspend the marketing authorisations of tetrazepam-containing medicines across the European Union (EU) in April 2013. The European Commission has confirmed the suspension of the marketing authorisations for Tetrazepam in Europe because of cutaneous toxicity, effective from the 1 August 2013.

Salicylate sensitivity is any adverse effect that occurs when a usual amount of salicylate is ingested. People with salicylate intolerance are unable to consume a normal amount of salicylate without adverse effects.
A drug allergy is an allergy to a drug, most commonly a medication, and is a form of adverse drug reaction. Medical attention should be sought immediately if an allergic reaction is suspected.
Drug reaction with eosinophilia and systemic symptoms (DRESS), also termed drug-induced hypersensitivity syndrome (DIHS), is a rare reaction to certain medications. It involves primarily a widespread skin rash, fever, swollen lymph nodes, and characteristic blood abnormalities such as an abnormally high level of eosinophils, low number of platelets, and increased number of atypical white blood cells (lymphocytes). However, DRESS is often complicated by potentially life-threatening inflammation of internal organs and the syndrome has about a 10% mortality rate. Treatment consists of stopping the offending medication and providing supportive care. Systemic corticosteroids are commonly used as well but no controlled clinical trials have assessed the efficacy of this treatment.
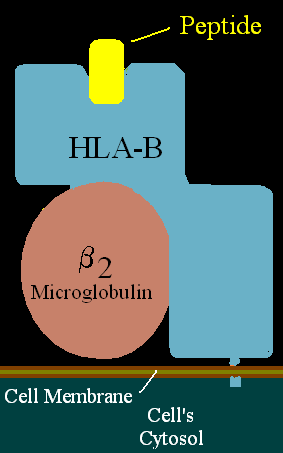
HLA-B58 (B58) is an HLA-B serotype. B58 is a split antigen from the B17 broad antigen, the sister serotype B57. The serotype identifies the more common HLA-B*58 gene products. B*5801 is associated with allopurinol induced inflammatory necrotic skin disease.
Anticonvulsant/sulfonamide hypersensitivity syndrome is a potentially serious hypersensitivity reaction that can be seen with medications with an aromatic amine chemical structure, such as aromatic anticonvulsants, sulfonamides, or other medications with an aromatic amine. Cross-reactivity should not occur between medications with an aromatic amine and medications without an aromatic amine ; therefore, these medications can be safely used in the future.

Acute generalized exanthematous pustulosis (AGEP) is a rare skin reaction that in 90% of cases is related to medication administration.
Serum sickness–like reactions (SSLRs) refer to adverse reactions that have symptoms similar to those of serum sickness but in which immune complexes are not found.
Fixed drug reactions, are common and so named because they recur at the same site with each exposure to a particular medication. Medications inducing fixed drug eruptions are usually those taken intermittently.
NSAID or nonsteroidal anti-inflammatory drug hypersensitivity reactions encompasses a broad range of allergic or allergic-like symptoms that occur within minutes to hours after ingesting aspirin or other NSAID nonsteroidal anti-inflammatory drugs. Hypersensitivity drug reactions differ from drug toxicity reactions in that drug toxicity reactions result from the pharmacological action of a drug, are dose-related, and can occur in any treated individual ; hypersensitivity reactions are idiosyncratic reactions to a drug. Although the term NSAID was introduced to signal a comparatively low risk of adverse effects, NSAIDs do evoke a broad range of hypersensitivity syndromes. These syndromes have recently been classified by the European Academy of Allergy and Clinical Immunology Task Force on NSAIDs Hypersensitivity.
Severe cutaneous adverse reactions are a group of potentially lethal adverse drug reactions that involve the skin and mucous membranes of various body openings such as the eyes, ears, and inside the nose, mouth, and lips. In more severe cases, SCARs also involves serious damage to internal organs. SCARs includes five syndromes: Drug reaction with eosinophilia and systemic symptoms ; Stevens–Johnson syndrome (SJS); Toxic epidermal necrolysis (TEN), Stevens-Johnson/toxic epidermal necrolysis overlap syndrome (SJS/TEN); and Acute generalized exanthematous pustulosis (AGEP). The five disorders have similar pathophysiologies, i.e. disease-causing mechanisms, for which new strategies are in use or development to identify individuals predisposed to develop the SCARs-inducing effects of specific drugs and thereby avoid treatment with them. Maculopapular rash (MPR) is a less-well defined and benign form of drug-induced adverse skin reactions; while not classified in the SCARs group, it shares with SCARS a similar pathophysiology and is caused by some of the same drugs which cause SCARs.
The p-i concept refers to the pharmacological interaction of drugs with immune receptors. It explains a form of drug hypersensitivity, namely T cell stimulation, which can lead to various acute inflammatory manifestations such as exanthems, eosinophilia and systemic symptoms, Stevens–Johnson syndrome, toxic epidermal nercrolysis, and complications upon withdrawing the drug.