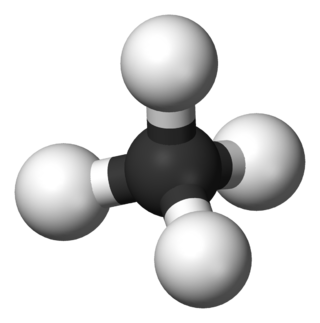
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic; their odor is usually faint, and may be similar to that of gasoline or lighter fluid. They occur in a diverse range of molecular structures and phases: they can be gases, liquids, low melting solids or polymers.

Natural gas is a naturally occurring mixture of gaseous hydrocarbons consisting primarily of methane (97%) in addition to various smaller amounts of other higher alkanes. Low levels of trace gases like carbon dioxide, nitrogen, hydrogen sulfide, and helium are also usually present. Methane is colorless and odorless, and the second largest greenhouse gas contributor to global climate change after carbon dioxide. Because natural gas is odorless, odorizers such as mercaptan are commonly added to it for safety so that leaks can be readily detected.

Pollution is the introduction of contaminants into the natural environment that cause adverse change. Pollution can take the form of any substance or energy. Pollutants, the components of pollution, can be either foreign substances/energies or naturally occurring contaminants.

A fossil fuel is a hydrocarbon-containing material such as coal, oil, and natural gas, formed naturally in the Earth's crust from the remains of dead plants and animals that is extracted and burned as a fuel. Fossil fuels may be burned to provide heat for use directly, to power engines, or to generate electricity. Some fossil fuels are refined into derivatives such as kerosene, gasoline and propane before burning. The origin of fossil fuels is the anaerobic decomposition of buried dead organisms, containing organic molecules created by photosynthesis. The conversion from these materials to high-carbon fossil fuels typically requires a geological process of millions of years.

Biogas is a gaseous renewable energy source produced from raw materials such as agricultural waste, manure, municipal waste, plant material, sewage, green waste, wastewater, and food waste. Biogas is produced by anaerobic digestion with anaerobic organisms or methanogens inside an anaerobic digester, biodigester or a bioreactor. The gas composition is primarily methane and carbon dioxide and may have small amounts of hydrogen sulfide, moisture and siloxanes. The methane can be combusted or oxidized with oxygen. This energy release allows biogas to be used as a fuel; it can be used in fuel cells and for heating purpose, such as in cooking. It can also be used in a gas engine to convert the energy in the gas into electricity and heat.

Biofuel is a fuel that is produced over a short time span from biomass, rather than by the very slow natural processes involved in the formation of fossil fuels such as oil. Biofuel can be produced from plants or from agricultural, domestic or industrial biowaste. Biofuels are mostly used for transportation, but can also be used for heating and electricity. Biofuels are regarded as a renewable energy source. The use of biofuel has been subject to criticism regarding the "food vs fuel" debate, varied assessments of their sustainability, and possible deforestation and biodiversity loss as a result of biofuel production.

Water pollution is the contamination of water bodies, usually as a result of human activities, that has a negative impact on their uses. Water bodies include lakes, rivers, oceans, aquifers, reservoirs and groundwater. Water pollution results when contaminants mix with these water bodies. Contaminants can come from one of four main sources: sewage discharges, industrial activities, agricultural activities, and urban runoff including stormwater. Water pollution is either surface water pollution or groundwater pollution. This form of pollution can lead to many problems, such as the degradation of aquatic ecosystems or spreading water-borne diseases when people use polluted water for drinking or irrigation. Another problem is that water pollution reduces the ecosystem services that the water resource would otherwise provide.

Alternative fuels, also known as non-conventional and advanced fuels, are fuels derived from sources other than petroleum. Alternative fuels include gaseous fossil fuels like propane, natural gas, methane, and ammonia; biofuels like biodiesel, bioalcohol, and refuse-derived fuel; and other renewable fuels like hydrogen and electricity.

A fossil fuel power station is a thermal power station which burns a fossil fuel, such as coal or natural gas, to produce electricity. Fossil fuel power stations have machinery to convert the heat energy of combustion into mechanical energy, which then operates an electrical generator. The prime mover may be a steam turbine, a gas turbine or, in small plants, a reciprocating gas engine. All plants use the energy extracted from the expansion of a hot gas, either steam or combustion gases. Although different energy conversion methods exist, all thermal power station conversion methods have their efficiency limited by the Carnot efficiency and therefore produce waste heat.

Petroleum coke, abbreviated coke, pet coke or petcoke, is a final carbon-rich solid material that derives from oil refining, and is one type of the group of fuels referred to as cokes. Petcoke is the coke that, in particular, derives from a final cracking process—a thermo-based chemical engineering process that splits long chain hydrocarbons of petroleum into shorter chains—that takes place in units termed coker units. Stated succinctly, coke is the "carbonization product of high-boiling hydrocarbon fractions obtained in petroleum processing ". Petcoke is also produced in the production of synthetic crude oil (syncrude) from bitumen extracted from Canada's tar sands and from Venezuela's Orinoco oil sands.

A gas flare, alternatively known as a flare stack, flare boom, ground flare, or flare pit, is a gas combustion device used in places such as petroleum refineries, chemical plants and natural gas processing plants, oil or gas extraction sites having oil wells, gas wells, offshore oil and gas rigs and landfills.
Renewable Fuels are fuels produced from renewable resources. Examples include: biofuels, Hydrogen fuel, and fully synthetic fuel produced from ambient carbon dioxide and water. This is in contrast to non-renewable fuels such as natural gas, LPG (propane), petroleum and other fossil fuels and nuclear energy. Renewable fuels can include fuels that are synthesized from renewable energy sources, such as wind and solar. Renewable fuels have gained in popularity due to their sustainability, low contributions to the carbon cycle, and in some cases lower amounts of greenhouse gases. The geo-political ramifications of these fuels are also of interest, particularly to industrialized economies which desire independence from Middle Eastern oil.

Algae fuel, algal biofuel, or algal oil is an alternative to liquid fossil fuels that uses algae as its source of energy-rich oils. Also, algae fuels are an alternative to commonly known biofuel sources, such as corn and sugarcane. When made from seaweed (macroalgae) it can be known as seaweed fuel or seaweed oil.
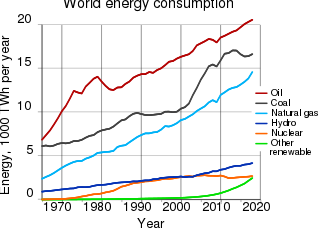
The environmental impact of the energy industry is significant, as energy and natural resource consumption are closely related. Producing, transporting, or consuming energy all have an environmental impact. Energy has been harnessed by human beings for millennia. Initially it was with the use of fire for light, heat, cooking and for safety, and its use can be traced back at least 1.9 million years. In recent years there has been a trend towards the increased commercialization of various renewable energy sources. Scientific consensus on some of the main human activities that contribute to global warming are considered to be increasing concentrations of greenhouse gases, causing a warming effect, global changes to land surface, such as deforestation, for a warming effect, increasing concentrations of aerosols, mainly for a cooling effect.

The environmental effects of shipping include air pollution, water pollution, acoustic, and oil pollution. Ships are responsible for more than 18% of nitrogen oxides pollution, and 3% of greenhouse gas emissions.

Individual action on climate change can include personal choices in many areas, such as diet, travel, household energy use, consumption of goods and services, and family size. Individuals can also engage in local and political advocacy around issues of climate change. People who wish to reduce their carbon footprint, can take "high-impact" actions, such as avoiding frequent flying and petrol fuelled cars, eating mainly a plant-based diet, having fewer children, using clothes and electrical products for longer, and electrifying homes. Avoiding meat and dairy foods has been called "the single biggest way" an individual can reduce their environmental impact. Excessive consumption is more to blame for climate change than population increase. High consumption lifestyles have a greater environmental impact, with the richest 10% of people emitting about half the total lifestyle emissions.

In an oil and gas production, flash-gas is a spontaneous vapor that is produced from the heating or depressurization of the extracted oil mixture during different phases of production. Flash evaporation, or flashing, is the process of volatile components suddenly vaporizing from their liquid state. This often happens during the transportation of petroleum products through pipelines and into vessels, such as when the stream from a common separation unit flows into an on-site atmospheric storage tank. Vessels that are used to intentionally “flash” a mixture of gas and saturated liquids are aptly named "flash drums." A type of vapor-liquid separator. A venting apparatus is used in these vessels to prevent damage due to increasing pressure, extreme cases of this are referred to as boiling liquid expanding vapor explosion (BLEVE).

Gas venting, more specifically known as natural-gas venting or methane venting, is the intentional and controlled release of gases containing alkane hydrocarbons - predominately methane - into Earth's atmosphere. It is a widely used method for disposal of unwanted gases which are produced during the extraction of coal and crude oil. Such gases may lack value when they are not recyclable into the production process, have no export route to consumer markets, or are surplus to near-term demand. In cases where the gases have value to the producer, substantial amounts may also be vented from the equipment used for gas collection, transport, and distribution.

Routine flaring, also known as production flaring, is a method and current practice of disposing of large unwanted amounts of associated petroleum gas (APG) during crude oil extraction. The gas is first separated from the liquids and solids downstream of the wellhead, then released into a flare stack and combusted into Earth's atmosphere. Where performed, the unwanted gas has been deemed unprofitable, and may be referred to as stranded gas, flare gas, or simply as "waste gas". Routine flaring is not to be confused with safety flaring, maintenance flaring, or other flaring practices characterized by shorter durations or smaller volumes of gas disposal.