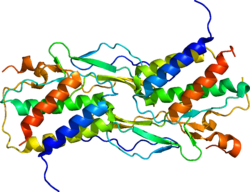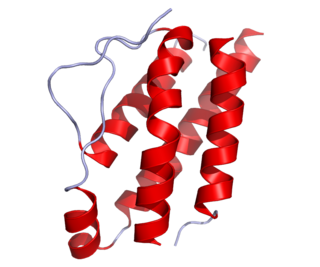
Interleukin-2 (IL-2) is an interleukin, a type of cytokine signaling molecule in the immune system. It is a 15.5–16 kDa protein that regulates the activities of white blood cells (leukocytes, often lymphocytes) that are responsible for immunity. IL-2 is part of the body's natural response to microbial infection, and in discriminating between foreign ("non-self") and "self". IL-2 mediates its effects by binding to IL-2 receptors, which are expressed by lymphocytes. The major sources of IL-2 are activated CD4+ T cells and activated CD8+ T cells. Put shortly the function of IL-2 is to stimulate the growth of helper, cytotoxic and regulatory T cells.

Interleukin 12 (IL-12) is an interleukin that is naturally produced by dendritic cells, macrophages, neutrophils, helper T cells and human B-lymphoblastoid cells (NC-37) in response to antigenic stimulation. IL-12 belongs to the family of interleukin-12. IL-12 family is unique in comprising the only heterodimeric cytokines, which includes IL-12, IL-23, IL-27 and IL-35. Despite sharing many structural features and molecular partners, they mediate surprisingly diverse functional effects.

The interleukin 4 is a cytokine that induces differentiation of naive helper T cells (Th0 cells) to Th2 cells. Upon activation by IL-4, Th2 cells subsequently produce additional IL-4 in a positive feedback loop. IL-4 is produced primarily by mast cells, Th2 cells, eosinophils and basophils. It is closely related and has functions similar to IL-13.
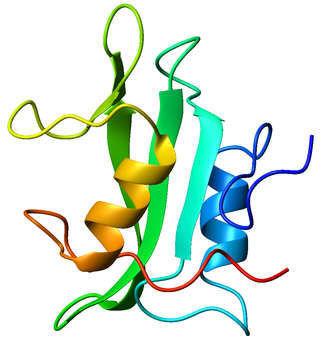
Lck is a 56 kDa protein that is found inside specialized cells of the immune system called lymphocytes. The Lck is a member of Src kinase family (SFK) and is important for the activation of T-cell receptor (TCR) signaling in both naive T cells and effector T cells. The role of Lck is less prominent in the activation or in the maintenance of memory CD8 T cells in comparison to CD4 T cells. In addition, the constitutive activity of the mouse Lck homolog varies among memory T cell subsets. It seems that in mice, in the effector memory T cell (TEM) population, more than 50% of Lck is present in a constitutively active conformation, whereas less than 20% of Lck is present as active form in central memory T cells. These differences are due to differential regulation by SH2 domain–containing phosphatase-1 (Shp-1) and C-terminal Src kinase.

IRAK-4, in the IRAK family, is a protein kinase involved in signaling innate immune responses from Toll-like receptors. It also supports signaling from T-cell receptors. IRAK4 contains domain structures which are similar to those of IRAK1, IRAK2, IRAKM and Pelle. IRAK4 is unique compared to IRAK1, IRAK2 and IRAKM in that it functions upstream of the other IRAKs, but is more similar to Pelle in this trait. IRAK4 has important clinical applications.

Interleukin 21 (IL-21) is a protein that in humans is encoded by the IL21 gene.

Interleukin 24 (IL-24) is a protein in the interleukin family, a type of cytokine signaling molecule in the immune system. In humans, this protein is encoded by the IL24 gene.

Interleukin 17 family is a family of pro-inflammatory cystine knot cytokines. They are produced by a group of T helper cell known as T helper 17 cell in response to their stimulation with IL-23. Originally, Th17 was identified in 1993 by Rouvier et al. who isolated IL17A transcript from a rodent T-cell hybridoma. The protein encoded by IL17A is a founding member of IL-17 family. IL17A protein exhibits a high homology with a viral IL-17-like protein encoded in the genome of T-lymphotropic rhadinovirus Herpesvirus saimiri. In rodents, IL-17A is often referred to as CTLA8.

The interleukin-1 receptor antagonist (IL-1RA) is a protein that in humans is encoded by the IL1RN gene.

Interleukin-1 receptor-associated kinase 1 (IRAK-1) is an enzyme in humans encoded by the IRAK1 gene. IRAK-1 plays an important role in the regulation of the expression of inflammatory genes by immune cells, such as monocytes and macrophages, which in turn help the immune system in eliminating bacteria, viruses, and other pathogens. IRAK-1 is part of the IRAK family consisting of IRAK-1, IRAK-2, IRAK-3, and IRAK-4, and is activated by inflammatory molecules released by signaling pathways during pathogenic attack. IRAK-1 is classified as a kinase enzyme, which regulates pathways in both innate and adaptive immune systems.
Interleukin 35 (IL-35) is a recently discovered anti-inflammatory cytokine from the IL-12 family. Member of IL-12 family - IL-35 is produced by wide range of regulatory lymphocytes and plays a role in immune suppression. IL-35 can block the development of Th1 and Th17 cells by limiting early T cell proliferation.

CD244 also known as 2B4 or SLAMF4 is a protein that in humans is encoded by the CD244 gene.
Signaling lymphocytic activation molecule 1 is a protein that in humans is encoded by the SLAMF1 gene. Recently SLAMF1 has also been designated CD150.

Interleukin-15 receptor is a type I cytokine receptor, binding interleukin-15.
Interleukin-28 receptor is a type II cytokine receptor found largely in epithelial cells. It binds type 3 interferons, interleukin-28 A, Interleukin-28B, interleukin 29 and interferon lambda 4. It consists of an α chain and shares a common β subunit with the interleukin-10 receptor. Binding to the interleukin-28 receptor, which is restricted to select cell types, is important for fighting infection. Binding of the type 3 interferons to the receptor results in activation of the JAK/STAT signaling pathway.

Interleukin-17A is a protein that in humans is encoded by the IL17A gene. In rodents, IL-17A used to be referred to as CTLA8, after the similarity with a viral gene.

The Interleukin-2 receptor alpha chain is a protein involved in the assembly of the high-affinity Interleukin-2 receptor, consisting of alpha (IL2RA), beta (IL2RB) and the common gamma chain (IL2RG). As the name indicates, this receptor interacts with Interleukin-2, a pleiotropic cytokine which plays an important role in immune homeostasis.
Interleukin 1 receptor-like 1, also known as IL1RL1 and ST2, is a protein that in humans is encoded by the IL1RL1 gene.

NKG2D is an activating receptor (transmembrane protein) belonging to the NKG2 family of C-type lectin-like receptors. NKG2D is encoded by KLRK1 (killer cell lectin like receptor K1) gene which is located in the NK-gene complex (NKC) situated on chromosome 6 in mice and chromosome 12 in humans. In mice, it is expressed by NK cells, NK1.1+ T cells, γδ T cells, activated CD8+ αβ T cells and activated macrophages. In humans, it is expressed by NK cells, γδ T cells and CD8+ αβ T cells. NKG2D recognizes induced-self proteins from MIC and RAET1/ULBP families which appear on the surface of stressed, malignant transformed, and infected cells.

CD28 family receptors are a group of regulatory cell surface receptors expressed on immune cells. The CD28 family in turn is a subgroup of the immunoglobulin superfamily.