Combinatorial chemistry comprises chemical synthetic methods that make it possible to prepare a large number of compounds in a single process. These compound libraries can be made as mixtures, sets of individual compounds or chemical structures generated by computer software. Combinatorial chemistry can be used for the synthesis of small molecules and for peptides.

A monoclonal antibody is an antibody made by cloning a unique white blood cell. All subsequent antibodies derived this way trace back to a unique parent cell.
Protein purification is a series of processes intended to isolate one or a few proteins from a complex mixture, usually cells, tissues or whole organisms. Protein purification is vital for the specification of the function, structure and interactions of the protein of interest. The purification process may separate the protein and non-protein parts of the mixture, and finally separate the desired protein from all other proteins. Separation of one protein from all others is typically the most laborious aspect of protein purification. Separation steps usually exploit differences in protein size, physico-chemical properties, binding affinity and biological activity. The pure result may be termed protein isolate.

Freeze drying, also known as lyophilisation or cryodesiccation, is a low temperature dehydration process that involves freezing the product, lowering pressure, then removing the ice by sublimation. This is in contrast to dehydration by most conventional methods that evaporate water using heat.

Purified water is water that has been mechanically filtered or processed to remove impurities and make it suitable for use. Distilled water was, formerly, the most common form of purified water, but, in recent years, water is more frequently purified by other processes including capacitive deionization, reverse osmosis, carbon filtering, microfiltration, ultrafiltration, ultraviolet oxidation, or electrodeionization. Combinations of a number of these processes have come into use to produce ultrapure water of such high purity that its trace contaminants are measured in parts per billion (ppb) or parts per trillion (ppt).
Affinity chromatography is a method of separating a biomolecule from a mixture, based on a highly specific macromolecular binding interaction between the biomolecule and another substance. The specific type of binding interaction depends on the biomolecule of interest; antigen and antibody, enzyme and substrate, receptor and ligand, or protein and nucleic acid binding interactions are frequently exploited for isolation of various biomolecules. Affinity chromatography is useful for its high selectivity and resolution of separation, compared to other chromatographic methods.

Ion exchange is a reversible interchange of one kind of ion present on an insoluble solid with another of like charge present in a solution surrounding the solid with the reaction being used especially for softening or making water demineralised, the purification of chemicals and separation of substances.

Ion chromatography separates ions and polar molecules based on their affinity to the ion exchanger. It works on almost any kind of charged molecule—including large proteins, small nucleotides, and amino acids. However, ion chromatography must be done in conditions that are one unit away from the isoelectric point of a protein.

Total organic carbon (TOC) is the amount of carbon found in an organic compound and is often used as a non-specific indicator of water quality or cleanliness of pharmaceutical manufacturing equipment. TOC may also refer to the amount of organic carbon in soil, or in a geological formation, particularly the source rock for a petroleum play; 2% is a rough minimum. For marine surface sediments average TOC content is 0.5% in the deep ocean, and 2% along the eastern margins.
Downstream processing refers to the recovery and the purification of biosynthetic products, particularly pharmaceuticals, from natural sources such as animal or plant tissue or fermentation broth, including the recycling of salvageable components and the proper treatment and disposal of waste. It is an essential step in the manufacture of pharmaceuticals such as antibiotics, hormones, antibodies and vaccines; antibodies and enzymes used in diagnostics; industrial enzymes; and natural fragrance and flavor compounds. Downstream processing is usually considered a specialized field in biochemical engineering, itself a specialization within chemical engineering, though many of the key technologies were developed by chemists and biologists for laboratory-scale separation of biological products.
In Ayurvedic medicine, the compilation of traditional ancient Indian medicine practice is called Rasa shastra, which details processes by which various metals, Minerals and other substances, including mercury, are purified and combined with herbs in an attempt to treat illnesses. Its methods correspond to the alchemy familiar in the Mediterranean and Western European worlds. Rasashastra is a pharmaceutical branch of Indian system of medicine which mainly deals with the metals, minerals, animal origin product, toxic herbs and their use in therapeutics.
Depyrogenation refers to the removal of pyrogens from solution, most commonly from injectable pharmaceuticals.

Basti is an important Shatkarma, a yogic purification, intended to clean the lower abdomen, especially the colon. The Hatha Yoga Pradipika and other sources attribute to it many beneficial effects. There are two ways to perform Basti:
Blood plasma fractionation refers to the general processes of separating the various components of blood plasma, which in turn is a component of blood obtained through blood fractionation. Plasma-derived immunoglobulins are giving a new narrative to healthcare across a wide range of autoimmune inflammatory diseases. This widespread applicability is anticipated to leverage market prospects for plasma fractionation, pegged to witness a noteworthy 7% CAGR. COVID-19 pandemic is expected to generate growth opportunities for the plasma fractionation market.
Reading Scientific Services Ltd. (RSSL) is a British company providing scientific analysis, consultancy, product development and training to the global food, drink, healthcare, pharmaceutical, biopharmaceutical and consumer goods sectors. It has been inspected by regulatory authorities including the U.S. Food and Drug Administration, the Medicines and Healthcare products Regulatory Agency and the United Kingdom Accreditation Service.
Ultrapure water (UPW), high-purity water or highly purified water (HPW) is water that has been purified to uncommonly stringent specifications. Ultrapure water is a term commonly used in the semiconductor industry to emphasize the fact that the water is treated to the highest levels of purity for all contaminant types, including: organic and inorganic compounds; dissolved and particulate matter; volatile and non-volatile; reactive, and inert; hydrophilic and hydrophobic; and dissolved gases.
Membrane technology covers all engineering approaches for the transport of substances between two fractions with the help of permeable membranes. In general, mechanical separation processes for separating gaseous or liquid streams use membrane technology.
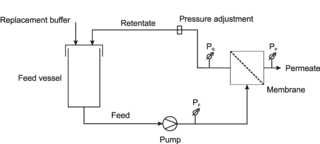
Diafiltration is a dilution process that involves removal or separation of components of a solution based on their molecular size by using micro-molecule permeable filters in order to obtain pure solution.
Host cell proteins (HCPs) are process-related protein impurities that are produced by the host organism during biotherapeutic manufacturing and production. During the purification process, a majority of produced HCPs are removed from the final product. However, residual HCPs still remain in the final distributed pharmaceutical drug. Examples of HCPs that may remain in the desired pharmaceutical product include: monoclonal antibodies (mAbs), antibody-drug-conjugates (ADCs), therapeutic proteins, vaccines, and other protein-based biopharmaceuticals.







