Dilationand curettage (D&C) refers to the dilation of the cervix and surgical removal of part of the lining of the uterus or contents of the uterus by scraping and scooping (curettage). It is a gynecologic procedure used for diagnostic and therapeutic purposes, and is the most commonly used method for first-trimester miscarriage or abortion.

Ectopic pregnancy is a complication of pregnancy in which the embryo attaches outside the uterus. Signs and symptoms classically include abdominal pain and vaginal bleeding, but fewer than 50 percent of affected women have both of these symptoms. The pain may be described as sharp, dull, or crampy. Pain may also spread to the shoulder if bleeding into the abdomen has occurred. Severe bleeding may result in a fast heart rate, fainting, or shock. With very rare exceptions, the fetus is unable to survive.
Sterilization is any of a number of medical methods of permanent birth control that intentionally leaves a person unable to reproduce. Sterilization methods include both surgical and non-surgical options for both males and females. Sterilization procedures are intended to be permanent; reversal is generally difficult.

Salpingectomy refers to the surgical removal of a fallopian tube. This may be done to treat an ectopic pregnancy or cancer, to prevent cancer, or as a form of contraception.
Dilation and evacuation (D&E) is the dilation of the cervix and surgical evacuation of the uterus after the first trimester of pregnancy. It is a method of abortion as well as a common procedure used after miscarriage to remove all pregnancy tissue.
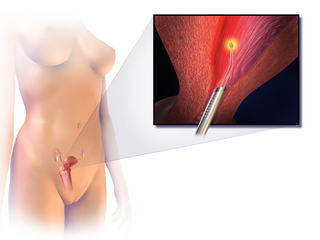
Endometrial ablation is a surgical procedure that is used to remove (ablate) or destroy the endometrial lining of the uterus. The goal of the procedure is to decrease the amount of blood loss during menstrual periods. Endometrial ablation is most often employed in people with excessive menstrual bleeding, who do not wish to undergo a hysterectomy, following unsuccessful medical therapy.

Essure was a device for female sterilization. It is a metal coil which when placed into each fallopian tube induces fibrosis and blockage. Essure was designed as an alternative to tubal ligation. However, it was recalled by Bayer in 2018, and the device is no longer sold due to complications secondary to its implantation. The company has reported that several patients implanted with the Essure System for Permanent Birth Control have experienced and/or reported adverse effects, including: perforation of the uterus and/or fallopian tubes, identification of inserts in the abdominal or pelvic cavity, persistent pain, and suspected allergic or hypersensitivity reaction. Although designed to remain in place for a lifetime, it was approved based on short-term safety studies. Of the 745 women with implants in the original premarket studies, 92% were followed up at one year, and 25% for two years, for safety outcomes. A 2009 review concluded that Essure appeared safe and effective based on short-term studies, that it was less invasive and could be cheaper than laparoscopic bilateral tubal ligation. About 750,000 women have received the device worldwide.

A hydrosalpinx is a condition that occurs when a fallopian tube is blocked and fills with serous or clear fluid near the ovary. The blocked tube may become substantially distended giving the tube a characteristic sausage-like or retort-like shape. The condition is often bilateral and the affected tubes may reach several centimeters in diameter. The blocked tubes cause infertility. A fallopian tube filled with blood is a hematosalpinx, and with pus a pyosalpinx.
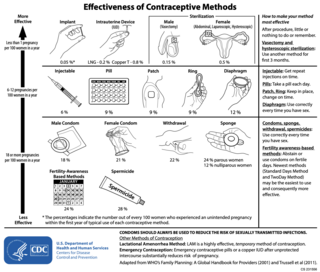
There are many methods of birth control that vary in requirements, side effects, and effectiveness. As the technology, education, and awareness about contraception has evolved, new contraception methods have been theorized and put in application. Although no method of birth control is ideal for every user, some methods remain more effective, affordable or intrusive than others. Outlined here are the different types of barrier methods, hormonal methods, various methods including spermicides, emergency contraceptives, and surgical methods and a comparison between them.
Tubal reversal, also called tubal sterilization reversal, tubal ligation reversal, or microsurgical tubal reanastomosis, is a surgical procedure that can restore fertility to women after a tubal ligation. By rejoining the separated segments of the fallopian tube, tubal reversal can give women the chance to become pregnant again. In some cases, however, the separated segments cannot actually be reattached to each other. In some cases the remaining segment of tube needs to be re-implanted into the uterus. In other cases, when the end of the tube has been removed, a procedure called a neofimbrioplasty must be performed to recreate a functional end of the tube which can then act like the missing fimbria and retrieve the egg that has been released during ovulation.

Fallopian tube obstruction, also known as fallopian tube occlusion, is a major cause of female infertility. Blocked fallopian tubes are unable to let the ovum and the sperm converge, thus making fertilization impossible.

Birth control, also known as contraception, anticonception, and fertility control, is the use of methods or devices to prevent unintended pregnancy. Birth control has been used since ancient times, but effective and safe methods of birth control only became available in the 20th century. Planning, making available, and using human birth control is called family planning. Some cultures limit or discourage access to birth control because they consider it to be morally, religiously, or politically undesirable.

The fallopian tubes, also known as uterine tubes, oviducts or salpinges, are paired tubes in the human female body that stretch from the uterus to the ovaries. The fallopian tubes are part of the female reproductive system. In other vertebrates, they are only called oviducts.
Reproductive surgery is surgery in the field of reproductive medicine. It can be used for contraception, e.g. in vasectomy, wherein the vasa deferentia of a male are severed, but is also used plentifully in assisted reproductive technology. Reproductive surgery is generally divided into three categories: surgery for infertility, in vitro fertilization, and fertility preservation.

An interstitial pregnancy is a uterine but ectopic pregnancy; the pregnancy is located outside the uterine cavity in that part of the fallopian tube that penetrates the muscular layer of the uterus. The term cornual pregnancy is sometimes used as a synonym, but remains ambiguous as it is also applied to indicate the presence of a pregnancy located within the cavity in one of the two upper "horns" of a bicornuate uterus. Interstitial pregnancies have a higher mortality than ectopics in general.
Endometriosis and its complications are a major cause of female infertility. Endometriosis is a dysfunction characterized by the migration of endometrial tissue to areas outside of the endometrium of the uterus. The most common places to find stray tissue are on ovaries and fallopian tubes, followed by other organs in the lower abdominal cavity such as the bladder and intestines. Typically, the endometrial tissue adheres to the exteriors of the organs, and then creates attachments of scar tissue called adhesions that can join adjacent organs together. The endometrial tissue and the adhesions can block a fallopian tube and prevent the meeting of ovum and sperm cells, or otherwise interfere with fertilization, implantation and, rarely, the carrying of the fetus to term.
High-grade serous carcinoma (HGSC) is a type of tumour that arises from the serous epithelial layer in the abdominopelvic cavity and is mainly found in the ovary. HGSCs make up the majority of ovarian cancer cases and have the lowest survival rates. HGSC is distinct from low-grade serous carcinoma (LGSC) which arises from ovarian tissue, is less aggressive and is present in stage I ovarian cancer where tumours are localised to the ovary.

Prophylactic salpingectomy is a preventative surgical technique performed on patients who are at higher risk of having ovarian cancer, such as individuals who may have pathogenic variants of the BRCA1 or BRCA2 gene. Originally salpingectomy was used in cases of ectopic pregnancies. As a preventative surgery however, it involves the removal of the fallopian tubes. By not removing the ovaries this procedure is advantageous to individuals who are still of child bearing age. It also reduces risks such as cardiovascular disease and osteoporosis which are associated with removal of the ovaries.
Chromopertubation is a method for the study of fallopian tube patency for suspected infertility in women caused by fallopian tube obstruction. Occlusion or pathology of the fallopian tubes is the most common cause of suspected infertility. Chromopertubation is sometimes commonly referred to a "laparoscopy and dye" test. It is currently one of the standard procedures in this field. In most cases, chromopertubation is performed to assess and determine the cause of someone's difficulties in getting pregnant.

The SEE-FIM protocol is a pathology dissection protocol for Sectioning and Extensively Examining the Fimbria (SEE-FIM). This protocol is intended to provide for the optimal microscopic examination of the distal fallopian tube (fimbria) to identify either cancerous or precancerous conditions in this organ.












