
Ytterbium is a chemical element; it has symbol Yb and atomic number 70. It is a metal, the fourteenth and penultimate element in the lanthanide series, which is the basis of the relative stability of its +2 oxidation state. Like the other lanthanides, its most common oxidation state is +3, as in its oxide, halides, and other compounds. In aqueous solution, like compounds of other late lanthanides, soluble ytterbium compounds form complexes with nine water molecules. Because of its closed-shell electron configuration, its density, melting point and boiling point are much lower than those of most other lanthanides.

Carbon tetrabromide, CBr4, also known as tetrabromomethane, is a bromide of carbon. Both names are acceptable under IUPAC nomenclature.

Vanadium(III) bromide, also known as vanadium tribromide, describes the inorganic compounds with the formula VBr3 and its hydrates. The anhydrous material is a green-black solid. In terms of its structure, the compound is polymeric with octahedral vanadium(III) surrounded by six bromide ligands.
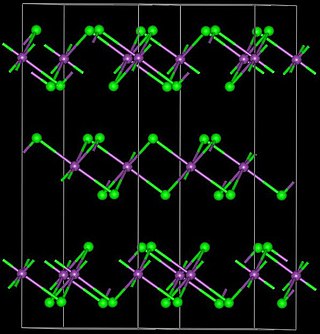
Ytterbium(III) bromide (YbBr3) is an inorganic chemical compound.
Tin(II) bromide is a chemical compound of tin and bromine with a chemical formula of SnBr2. Tin is in the +2 oxidation state. The stability of tin compounds in this oxidation state is attributed to the inert pair effect.
Bromine compounds are compounds containing the element bromine (Br). These compounds usually form the -1, +1, +3 and +5 oxidation states. Bromine is intermediate in reactivity between chlorine and iodine, and is one of the most reactive elements. Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine. This can be seen from the standard electrode potentials of the X2/X− couples (F, +2.866 V; Cl, +1.395 V; Br, +1.087 V; I, +0.615 V; At, approximately +0.3 V). Bromination often leads to higher oxidation states than iodination but lower or equal oxidation states to chlorination. Bromine tends to react with compounds including M–M, M–H, or M–C bonds to form M–Br bonds.
There are three sets of Indium halides, the trihalides, the monohalides, and several intermediate halides. In the monohalides the oxidation state of indium is +1 and their proper names are indium(I) fluoride, indium(I) chloride, indium(I) bromide and indium(I) iodide.

Bromopentaamminecobalt(III) bromide is the dibromide salt of the cobalt coordination compound with the formula [Co(NH3)5Br]2+. It is a purple, water-soluble solid. The analogous chloropentaamminecobalt(III) chloride is also well known.

Chromium(III) bromide is an inorganic compound with the chemical formula CrBr3. It is a dark colored solid that appears green in transmitted light but red with reflected light. It is used as a precursor to catalysts for the oligomerization of ethylene.
Titanium(III) bromide is the inorganic compound with the formula TiBr3. It is a blue black paramagnetic solid with a reddish reflection. It has few applications, although it is a catalyst for the polymerization of alkenes.

Chromium(II) bromide is the inorganic compound with the chemical formula CrBr2. Like many metal dihalides, CrBr2 adopts the "cadmium iodide structure" motif, i.e., it features sheets of octahedral Cr(II) centers interconnected by bridging bromide ligands. It is a white solid that dissolves in water to give blue solutions that are readily oxidized by air.
Nickel is one of the metals that can form Tutton's salts. The singly charged ion can be any of the full range of potassium, rubidium, cesium, ammonium (), or thallium. As a mineral the ammonium nickel salt, (NH4)2Ni(SO4)2 · 6 H2O, can be called nickelboussingaultite. With sodium, the double sulfate is nickelblödite Na2Ni(SO4)2 · 4 H2O from the blödite family. Nickel can be substituted by other divalent metals of similar sized to make mixtures that crystallise in the same form.

Europium(II) chloride is an inorganic compound with a chemical formula EuCl2. When it is irradiated by ultraviolet light, it has bright blue fluorescence.
Arsenide bromides or bromide arsenides are compounds containing anions composed of bromide (Br−) and arsenide (As3−). They can be considered as mixed anion compounds. They are in the category of pnictidehalides. Related compounds include the arsenide chlorides, arsenide iodides, phosphide bromides, and antimonide bromides.

Neodymium(II) bromide is an inorganic compound of neodymium and bromide.

Europium compounds are compounds formed by the lanthanide metal europium (Eu). In these compounds, europium generally exhibits the +3 oxidation state, such as EuCl3, Eu(NO3)3 and Eu(CH3COO)3. Compounds with europium in the +2 oxidation state are also known. The +2 ion of europium is the most stable divalent ion of lanthanide metals in aqueous solution. Many europium compounds fluoresce under ultraviolet light due to the excitation of electrons to higher energy levels. Lipophilic europium complexes often feature acetylacetonate-like ligands, e.g., Eufod.
Ytterbium compounds are chemical compounds that contain the element ytterbium (Yb). The chemical behavior of ytterbium is similar to that of the rest of the lanthanides. Most ytterbium compounds are found in the +3 oxidation state, and its salts in this oxidation state are nearly colorless. Like europium, samarium, and thulium, the trihalides of ytterbium can be reduced to the dihalides by hydrogen, zinc dust, or by the addition of metallic ytterbium. The +2 oxidation state occurs only in solid compounds and reacts in some ways similarly to the alkaline earth metal compounds; for example, ytterbium(II) oxide (YbO) shows the same structure as calcium oxide (CaO).
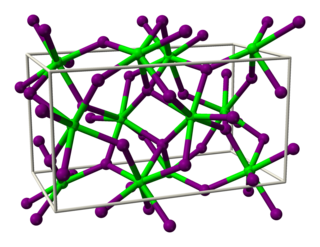
Thulium dibromide is an inorganic compound, with the chemical formula of TmBr2. It is a dark green solid that is easy to dissolve, with the SrI2 structure and it needs to be stored in an inert atmosphere.
Ytterbium monotelluride is an inorganic compound, one of the tellurides of ytterbium, with the chemical formula YbTe.









