
Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula BN. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexagonal form corresponding to graphite is the most stable and soft among BN polymorphs, and is therefore used as a lubricant and an additive to cosmetic products. The cubic variety analogous to diamond is called c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare wurtzite BN modification is similar to lonsdaleite but slightly softer than the cubic form.

Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three valence electrons for forming covalent bonds, resulting in many compounds such as boric acid, the mineral sodium borate, and the ultra-hard crystals of boron carbide and boron nitride.

Scandium is a chemical element with the symbol Sc and atomic number 21. It is a silvery-white metallic d-block element. Historically, it has been classified as a rare-earth element, together with yttrium and the lanthanides. It was discovered in 1879 by spectral analysis of the minerals euxenite and gadolinite from Scandinavia.
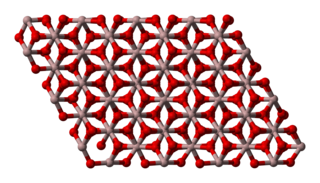
Aluminium oxide (or Aluminium(III) oxide) is a chemical compound of aluminium and oxygen with the chemical formula Al2O3. It is the most commonly occurring of several aluminium oxides, and specifically identified as aluminium oxide. It is commonly called alumina and may also be called aloxide, aloxite, or alundum in various forms and applications. It occurs naturally in its crystalline polymorphic phase α-Al2O3 as the mineral corundum, varieties of which form the precious gemstones ruby and sapphire. Al2O3 is significant in its use to produce aluminium metal, as an abrasive owing to its hardness, and as a refractory material owing to its high melting point.

Boron carbide (chemical formula approximately B4C) is an extremely hard boron–carbon ceramic, a covalent material used in tank armor, bulletproof vests, engine sabotage powders, as well as numerous industrial applications. With a Vickers hardness of >30 GPa, it is one of the hardest known materials, behind cubic boron nitride and diamond.
A superhard material is a material with a hardness value exceeding 40 gigapascals (GPa) when measured by the Vickers hardness test. They are virtually incompressible solids with high electron density and high bond covalency. As a result of their unique properties, these materials are of great interest in many industrial areas including, but not limited to, abrasives, polishing and cutting tools, disc brakes, and wear-resistant and protective coatings.

Aluminium sulfide is a chemical compound with the formula Al2S3. This colorless species has an interesting structural chemistry, existing in several forms. The material is sensitive to moisture, hydrolyzing to hydrated aluminum oxides/hydroxides. This can begin when the sulfide is exposed to the atmosphere. The hydrolysis reaction generates gaseous hydrogen sulfide (H2S).

Aluminium diboride (AlB2) is a chemical compound made from the metal aluminium and the metalloid boron. It is one of two compounds of aluminium and boron, the other being AlB12, which are both commonly referred to as aluminium boride.
Aluminium magnesium boride or Al3Mg3B56, colloquially known as BAM, is a chemical compound of aluminium, magnesium and boron. Whereas its nominal formula is AlMgB14, the chemical composition is closer to Al0.75Mg0.75B14. It is a ceramic alloy that is highly resistive to wear and has an extremely low coefficient of sliding friction, reaching a record value of 0.04 in unlubricated and 0.02 in lubricated AlMgB14−TiB2 composites. First reported in 1970, BAM has an orthorhombic structure with four icosahedral B12 units per unit cell. This ultrahard material has a coefficient of thermal expansion comparable to that of other widely used materials such as steel and concrete.

Barium borate is an inorganic compound, a borate of barium with a chemical formula BaB2O4 or Ba(BO2)2. It is available as a hydrate or dehydrated form, as white powder or colorless crystals. The crystals exist in the high-temperature α phase and low-temperature β phase, abbreviated as BBO; both phases are birefringent, and BBO is a common nonlinear optical material.

Yttrium boride refers to a crystalline material composed of different proportions of yttrium and boron, such as YB2, YB4, YB6, YB12, YB25, YB50 and YB66. They are all gray-colored, hard solids having high melting temperatures. The most common form is the yttrium hexaboride YB6. It exhibits superconductivity at relatively high temperature of 8.4 K and, similar to LaB6, is an electron cathode. Another remarkable yttrium boride is YB66. It has a large lattice constant (2.344 nm), high thermal and mechanical stability, and therefore is used as a diffraction grating for low-energy synchrotron radiation (1–2 keV).

Osmium borides are compounds of osmium and boron. Their most remarkable property is potentially high hardness. It is thought that a combination of high electron density of osmium with the strength of boron-osmium covalent bonds will make osmium borides superhard materials, however this has not been demonstrated yet. For example, OsB2 is hard (hardness comparable to that of sapphire), but not superhard.

Tungsten borides are compounds of tungsten and boron. Their most remarkable property is high hardness. The Vickers hardness of WB or WB2 crystals is ~20 GPa and that of WB4 is ~30 GPa for loads exceeding 3 N.

Boron can be prepared in several crystalline and amorphous forms. Well known crystalline forms are α-rhombohedral (α-R), β-rhombohedral (β-R), and β-tetragonal (β-T). In special circumstances, boron can also be synthesized in the form of its α-tetragonal (α-T) and γ-orthorhombic (γ) allotropes. Two amorphous forms, one a finely divided powder and the other a glassy solid, are also known. Although at least 14 more allotropes have been reported, these other forms are based on tenuous evidence or have not been experimentally confirmed, or are thought to represent mixed allotropes, or boron frameworks stabilized by impurities. Whereas the β-rhombohedral phase is the most stable and the others are metastable, the transformation rate is negligible at room temperature, and thus all five phases can exist at ambient conditions. Amorphous powder boron and polycrystalline β-rhombohedral boron are the most common forms. The latter allotrope is a very hard grey material, about ten percent lighter than aluminium and with a melting point (2080 °C) several hundred degrees higher than that of steel.
Metals, and specifically rare-earth elements, form numerous chemical complexes with boron. Their crystal structure and chemical bonding depend strongly on the metal element M and on its atomic ratio to boron. When B/M ratio exceeds 12, boron atoms form B12 icosahedra which are linked into a three-dimensional boron framework, and the metal atoms reside in the voids of this framework. Those icosahedra are basic structural units of most allotropes of boron and boron-rich rare-earth borides. In such borides, metal atoms donate electrons to the boron polyhedra, and thus these compounds are regarded as electron-deficient solids.
Boron steel refers to steel alloyed with a small amount of boron, usually less than 1%. The addition of boron to steel greatly increases the hardenability of the resulting alloy.

Gallium(III) sulfide, Ga2S3, is a compound of sulfur and gallium, that is a semiconductor that has applications in electronics and photonics.

Iron boride refers to various inorganic compounds with the formula FexBy. Two main iron borides are FeB and Fe2B. Some iron borides possess useful properties such as magnetism, electrical conductivity, corrosion resistance and extreme hardness. Some iron borides have found use as hardening coatings for iron. Iron borides have properties of ceramics such as high hardness, and properties of metal properties, such as thermal conductivity and electrical conductivity. Boride coatings on iron are superior mechanical, frictional, and anti-corrosive. Iron monoboride (FeB) is a grey powder that is insoluble in water. FeB is harder than Fe2B, but is more brittle and more easily fractured upon impact.
Boron carbides are boron–carbon compounds.

Aluminium (or aluminum) combines characteristics of pre- and post-transition metals. Since it has few available electrons for metallic bonding, like its heavier group 13 congeners, it has the characteristic physical properties of a post-transition metal, with longer-than-expected interatomic distances. Furthermore, as Al3+ is a small and highly charged cation, it is strongly polarizing and aluminium compounds tend towards covalency; this behaviour is similar to that of beryllium (Be2+), an example of a diagonal relationship. However, unlike all other post-transition metals, the underlying core under aluminium's valence shell is that of the preceding noble gas, whereas for gallium and indium it is that of the preceding noble gas plus a filled d-subshell, and for thallium and nihonium it is that of the preceding noble gas plus filled d- and f-subshells. Hence, aluminium does not suffer the effects of incomplete shielding of valence electrons by inner electrons from the nucleus that its heavier congeners do. Aluminium's electropositive behavior, high affinity for oxygen, and highly negative standard electrode potential are all more similar to those of scandium, yttrium, lanthanum, and actinium, which have ds2 configurations of three valence electrons outside a noble gas core: aluminium is the most electropositive metal in its group. Aluminium also bears minor similarities to the metalloid boron in the same group; AlX3 compounds are valence isoelectronic to BX3 compounds (they have the same valence electronic structure), and both behave as Lewis acids and readily form adducts. Additionally, one of the main motifs of boron chemistry is regular icosahedral structures, and aluminium forms an important part of many icosahedral quasicrystal alloys, including the Al–Zn–Mg class.















