Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula Li[AlH4] or LiAlH4. It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters, carboxylic acids, and amides. The solid is dangerously reactive toward water, releasing gaseous hydrogen (H2). Some related derivatives have been discussed for hydrogen storage.

Aluminium sulfate is a salt with the formula Al2(SO4)3. It is soluble in water and is mainly used as a coagulating agent (promoting particle collision by neutralizing charge) in the purification of drinking water and wastewater treatment plants, and also in paper manufacturing.
Titanium(III) chloride is the inorganic compound with the formula TiCl3. At least four distinct species have this formula; additionally hydrated derivatives are known. TiCl3 is one of the most common halides of titanium and is an important catalyst for the manufacture of polyolefins.
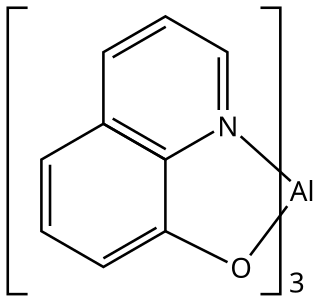
Tris(8-hydroxyquinolinato)aluminium is the chemical compound with the formula Al(C9H6NO)3. Widely abbreviated Alq3, it is a coordination complex wherein aluminium is bonded in a bidentate manner to the conjugate base of three 8-hydroxyquinoline ligands.

Chromium(III) acetylacetonate is the coordination compound with the formula Cr(C5H7O2)3, sometimes designated as Cr(acac)3. This purplish coordination complex is used in NMR spectroscopy as a relaxation agent because of its solubility in nonpolar organic solvents and its paramagnetism.
Organovanadium chemistry is the chemistry of organometallic compounds containing a carbon (C) to vanadium (V) chemical bond. Organovanadium compounds find only minor use as reagents in organic synthesis but are significant for polymer chemistry as catalysts.

Nickel(II) bis(acetylacetonate) is a coordination complex with the formula [Ni(acac)2]3, where acac is the anion C5H7O2− derived from deprotonation of acetylacetone. It is a dark green paramagnetic solid that is soluble in organic solvents such as toluene. It reacts with water to give the blue-green diaquo complex Ni(acac)2(H2O)2.

Ruthenium(III) acetylacetonate is a coordination complex with the formula Ru(O2C5H7)3. O2C5H7− is the ligand called acetylacetonate. This compound exists as a dark violet solid that is soluble in most organic solvents. It is used as a precursor to other compounds of ruthenium.

Tris(acetylacetonato) iron(III), often abbreviated Fe(acac)3, is a ferric coordination complex featuring acetylacetonate (acac) ligands, making it one of a family of metal acetylacetonates. It is a red air-stable solid that dissolves in nonpolar organic solvents.
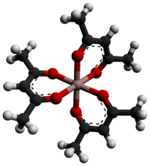
Metal acetylacetonates are coordination complexes derived from the acetylacetonate anion (CH
3COCHCOCH−
3) and metal ions, usually transition metals. The bidentate ligand acetylacetonate is often abbreviated acac. Typically both oxygen atoms bind to the metal to form a six-membered chelate ring. The simplest complexes have the formula M(acac)3 and M(acac)2. Mixed-ligand complexes, e.g. VO(acac)2, are also numerous. Variations of acetylacetonate have also been developed with myriad substituents in place of methyl (RCOCHCOR′−). Many such complexes are soluble in organic solvents, in contrast to the related metal halides. Because of these properties, acac complexes are sometimes used as catalyst precursors and reagents. Applications include their use as NMR "shift reagents" and as catalysts for organic synthesis, and precursors to industrial hydroformylation catalysts. C
5H
7O−
2 in some cases also binds to metals through the central carbon atom; this bonding mode is more common for the third-row transition metals such as platinum(II) and iridium(III).

Vanadyl acetylacetonate is the chemical compound with the formula VO(acac)2, where acac– is the conjugate base of acetylacetone. It is a blue-green solid that dissolves in polar organic solvents. The coordination complex consists of the vanadyl group, VO2+, bound to two acac– ligands via the two oxygen atoms on each. Like other charge-neutral acetylacetonate complexes, it is not soluble in water.
Barium acetylacetonate is a compound with formula Ba(C5H7O2)2. It is the Ba2+ complex of the anion acetylacetonate. The compound is typically encountered as an ill-defined hydrate, which would accord with the high coordination number characteristic of barium.

Dysprosium acetylacetonate is a chemical compound of dysprosium with formula Dy(C5H7O2)3(H2O)n.

Europium acetylacetonate is a compound with formula Eu(C5H7O2)3(H2O)2. It is a europium(III) complex with three acetylacetonate and two aquo ligands. The electronic structure of the Eu3+
core gives the complex an unusual charge-transfer band absent in other lanthanide acetylacetonates. The photoluminescent emission lines occur near 465 (blue), 525 (green), and 579 nm (yellow), and are unusually sharp, especially the yellow doublet. Doping a blend of polyacrylate and polycarbonate with europium acetylacetonate enhances photoluminescence over a broad range of ultraviolet wavelengths. EuFOD is a substituted derivative.
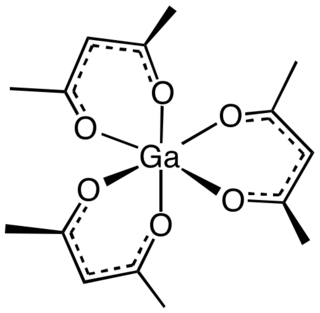
Gallium acetylacetonate, also referred to as Ga(acac)3, is a coordination complex with formula Ga(C5H7O2)3. This gallium complex with three acetylacetonate ligands is used in research. The molecule has D3 symmetry, being isomorphous with other octahedral tris(acetylacetonate)s.
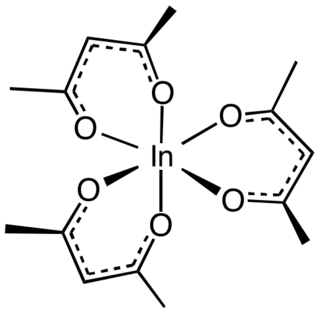
Indium acetylacetonate, also known as In(acac)3, is a compound with formula In(C5H7O2)3. It is a colorless solid. It adopts an octahedral structure.
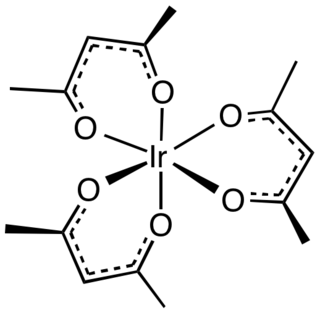
Iridium acetylacetonate is the iridium coordination complex with the formula Ir(O2C5H7)3, which is sometimes known as Ir(acac)3. The molecule has D3-symmetry. It is a yellow-orange solid that is soluble in organic solvents.
Aluminium triacetate, formally named aluminium acetate, is a chemical compound with composition Al(CH
3CO
2)
3. Under standard conditions it appears as a white, water-soluble solid that decomposes on heating at around 200 °C. The triacetate hydrolyses to a mixture of basic hydroxide / acetate salts, and multiple species co-exist in chemical equilibrium, particularly in aqueous solutions of the acetate ion; the name aluminium acetate is commonly used for this mixed system.
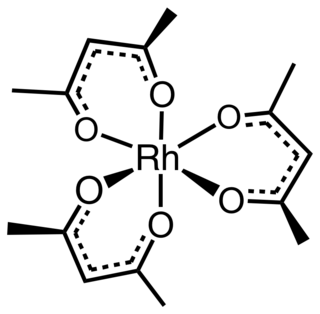
Rhodium acetylacetonate is the coordination complex with the formula Rh(C5H7O2)3, which is sometimes known as Rh(acac)3. The molecule has D3-symmetry. It is a yellow-orange solid that is soluble in organic solvents.

Tris(acetylacetonato)cobalt(III) is the coordination complex with the formula Co(C5H7O2)3. Often abbreviated Co(acac)3, it is a green, diamagnetic solid that is soluble in organic solvents, but not in water. Owing to its solubility in organic solvents, tris(acetylacetonato)cobalt(III) is used to produce homogeneous catalysts by reduction.