
Terbium is a chemical element; it has symbol Tb and atomic number 65. It is a silvery-white, rare earth metal that is malleable, and ductile. The ninth member of the lanthanide series, terbium is a fairly electropositive metal that reacts with water, evolving hydrogen gas. Terbium is never found in nature as a free element, but it is contained in many minerals, including cerite, gadolinite, monazite, xenotime and euxenite.

Terbium(III) bromide (TbBr3) is a crystalline chemical compound.

Terbium(III) chloride (TbCl3) is a chemical compound. In the solid state TbCl3 has the YCl3 layer structure. Terbium(III) chloride frequently forms a hexahydrate.

Nickel(II) bis(acetylacetonate) is a coordination complex with the formula [Ni(acac)2]3, where acac is the anion C5H7O2− derived from deprotonation of acetylacetone. It is a dark green paramagnetic solid that is soluble in organic solvents such as toluene. It reacts with water to give the blue-green diaquo complex Ni(acac)2(H2O)2.

Tris(acetylacetonato) iron(III), often abbreviated Fe(acac)3, is a ferric coordination complex featuring acetylacetonate (acac) ligands, making it one of a family of metal acetylacetonates. It is a red air-stable solid that dissolves in nonpolar organic solvents.
Metal acetylacetonates are coordination complexes derived from the acetylacetonate anion (CH
3COCHCOCH−
3) and metal ions, usually transition metals. The bidentate ligand acetylacetonate is often abbreviated acac. Typically both oxygen atoms bind to the metal to form a six-membered chelate ring. The simplest complexes have the formula M(acac)3 and M(acac)2. Mixed-ligand complexes, e.g. VO(acac)2, are also numerous. Variations of acetylacetonate have also been developed with myriad substituents in place of methyl (RCOCHCOR′−). Many such complexes are soluble in organic solvents, in contrast to the related metal halides. Because of these properties, acac complexes are sometimes used as catalyst precursors and reagents. Applications include their use as NMR "shift reagents" and as catalysts for organic synthesis, and precursors to industrial hydroformylation catalysts. C
5H
7O−
2 in some cases also binds to metals through the central carbon atom; this bonding mode is more common for the third-row transition metals such as platinum(II) and iridium(III).

Dysprosium acetylacetonate is a chemical compound of dysprosium with formula Dy(C5H7O2)3(H2O)n.

Europium acetylacetonate is a coordination complex with formula Eu(C5H7O2)3. Although this anhydrous acetylacetonate complex is widel discussed, some sources suggest that it is really the dihydrate Eu(C5H7O2)3(H2O)2.

Gadolinium acetylacetonate is a coordination compound with the formula Gd(C5H7O2)3. This anhydrous acetylacetonate complex is widely discussed but unlikely to exist per se. The 8-coordinated dihydrate Gd(C5H7O2)3(H2O)2 is a more plausible formula based on the behavior of other lathanide complexes. It has also been characterized twice by X-ray crystallography.

Neodymium(III) acetylacetonate is a coordination compound with the chemical formula Nd(O2C5H7)3. Although many sources discuss this anhydrous acetylacetonate complex, it is the dihydrate Nd(O2C5H7)3(H2O)2 that has been characterized by X-ray crystallography. It commonly occurs as a white powder. Upon heating under vacuum, other dihydrated lanthanide trisacetylacetonates convert to oxo-clusters M4O(C5H7O2)10. This result suggests that Nd(O2C5H7)3 may not exist.

Holmium acetylacetonate is a coordination compound with the formula Ho(C5H7O2)3. This anhydrous acetylacetonate complex is often discussed but unlikely to exist per se. The 8-coordinated dihydrate Ho(C5H7O2)3(H2O)2 is a more plausible formula based on the behavior of other lanthanide acetylacetonates. The dihydrate has been characterized by X-ray crystallography.

Terbium compounds are compounds formed by the lanthanide metal terbium (Tb). Terbium generally exhibits the +3 oxidation state in these compounds, such as in TbCl3, Tb(NO3)3 and Tb(CH3COO)3. Compounds with terbium in the +4 oxidation state are also known, such as TbO2 and BaTbF6. Terbium can also form compounds in the 0, +1 and +2 oxidation states.
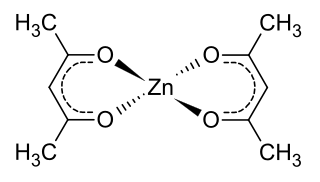
Zinc acetylacetonate is an acetylacetonate complex of zinc, with the chemical formula of Zn(C5H7O2)2. The compound is in fact a trimer, Zn3(acac)6, in which each Zn ion is coordinated by five oxygen atoms in a distorted trigonal bipyramidal structure.

Yttrium acetylacetonate is a coordination compound with the chemical formula Y(C5H7O2)3(H2O)x, or Y(acac)3(H2O)x for short. The value of x can vary from 1 to 3.

Praseodymium acetylacetonate is a coordination complex with the formula Pr(C3H7O2)3. This purported anhydrous acetylacetonate complex is widely discussed but only the dihydrate Pr(C3H7O2)3(H2O)2 has been characterized by X-ray crystallography.

Erbium acetylacetonate is a coordination compound with the formula Er(C5H7O2)3. This anhydrous acetylacetonate complex is often discussed but unlikely to exist per se. The 8-coordinated dihydrate Er(C5H7O2)3(H2O)2 is a more plausible formula based on the behavior of other lanthanide acetylacetonates. The dihydrate has been characterized by X-ray crystallography.

Thulium acetylacetonate is a coordination compound with the formula Tm(C5H7O2)3. This anhydrous acetylacetonate complex is often discussed but unlikely to exist per se. The 8-coordinated dihydrate Tm(C5H7O2)3(H2O)2 is a more plausible formula based on the behavior of other lanthanide acetylacetonates. The dihydrate has been characterized by X-ray crystallography. Upon attempted dehydration by heating under vacuum, other hydrated lanthanide tris(acetylacetonate) complexes decompose to give oxo-clusters.

Lanthanum acetylacetonate refers to the coordination complex with the formula La(C5H7O2)3. This anhydrous acetylacetonate complex has not been characterized well, but the dihydrate La(C5H7O2)3(H2O)2 has been characterized by X-ray crystallography.

Samarium acetylacetonate is a coordination compound with the formula Sm(C5H7O2)3. This anhydrous acetylacetonate complex is widely discussed but unlikely to exist per se. The 8-coordinated dihydrate Sm(C5H7O2)3(H2O)2 is a more plausible formula based on the behavior of other lanthanide acetylacetonates. The dihydrate has been characterized by X-ray crystallography. Upon attempted dehydration by heating under vacuum, other hydrated lanthanide tris(acetylacetonate) complexes decompose to give oxo-clusters.

Lutetium acetylacetonate is a coordination compound with the chemical formula Lu(C5H7O2)3, or Lu(acac)3 for short. The complex per se is unlikely to exist, but the dihydrate would be expected based on the behavior of other lanthanide tris(acetylacetonate)s. Consistent with this scenario, It forms adducts Lu(acac)3(phen) and Lu(acac)3(dipy) where phen and bipy are 1,10-phenanthroline and 2,2'-bipyridine, respectively.



















