
Holmium is a chemical element; it has symbol Ho and atomic number 67. It is a rare-earth element and the eleventh member of the lanthanide series. It is a relatively soft, silvery, fairly corrosion-resistant and malleable metal. Like many other lanthanides, holmium is too reactive to be found in native form, as pure holmium slowly forms a yellowish oxide coating when exposed to air. When isolated, holmium is relatively stable in dry air at room temperature. However, it reacts with water and corrodes readily, and also burns in air when heated.
The lanthanide or lanthanoid series of chemical elements comprises at least the 14 metallic chemical elements with atomic numbers 57–70, from lanthanum through ytterbium. In the periodic table, they fill the 4f orbitals. Lutetium is also sometimes considered a lanthanide, despite being a d-block element and a transition metal.

Thulium is a chemical element; it has symbol Tm and atomic number 69. It is the thirteenth and third-last element in the lanthanide series. Like the other lanthanides, the most common oxidation state is +3, seen in its oxide, halides and other compounds; however, the +2 oxidation state can also be stable. In aqueous solution, like compounds of other late lanthanides, soluble thulium compounds form coordination complexes with nine water molecules.
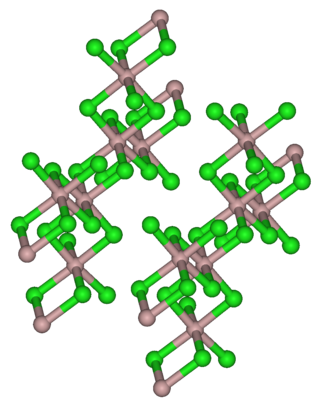
Thulium(III) chloride or thulium trichloride is as an inorganic salt composed of thulium and chlorine with the formula TmCl3. It forms yellow crystals. Thulium(III) chloride has the YCl3 (AlCl3) layer structure with octahedral thulium ions. It has been used as a starting material for some exotic nanostructures prepared for NIR photocatalysis.

Dysprosium acetylacetonate is a chemical compound of dysprosium with formula Dy(C5H7O2)3(H2O)n.

Europium acetylacetonate is a coordination complex with formula Eu(C5H7O2)3. Although this anhydrous acetylacetonate complex is widel discussed, some sources suggest that it is really the dihydrate Eu(C5H7O2)3(H2O)2.

Gadolinium acetylacetonate is a coordination compound with the formula Gd(C5H7O2)3. This anhydrous acetylacetonate complex is widely discussed but unlikely to exist per se. The 8-coordinated dihydrate Gd(C5H7O2)3(H2O)2 is a more plausible formula based on the behavior of other lathanide complexes. It has also been characterized twice by X-ray crystallography.
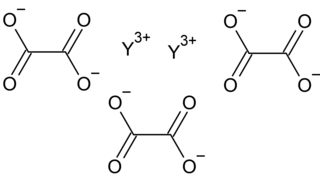
Yttrium oxalate is an inorganic compound, a salt of yttrium and oxalic acid with the chemical formula Y2(C2O4)3. The compound does not dissolve in water and forms crystalline hydrates—colorless crystals.

Thulium(III) nitrate is an inorganic compound, a salt of thulium and nitric acid with the chemical formula Tm(NO3)3. The compound forms dark-green crystals, readily soluble in water, also forms crystalline hydrates.

Neodymium(II) iodide or neodymium diiodide is an inorganic salt of iodine and neodymium the formula NdI2. Neodymium uses the +2 oxidation state in the compound.

Neodymium(III) acetylacetonate is a coordination compound with the chemical formula Nd(O2C5H7)3. Although many sources discuss this anhydrous acetylacetonate complex, it is the dihydrate Nd(O2C5H7)3(H2O)2 that has been characterized by X-ray crystallography. It commonly occurs as a white powder. Upon heating under vacuum, other dihydrated lanthanide trisacetylacetonates convert to oxo-clusters M4O(C5H7O2)10. This result suggests that Nd(O2C5H7)3 may not exist.

Holmium acetylacetonate is a coordination compound with the formula Ho(C5H7O2)3. This anhydrous acetylacetonate complex is often discussed but unlikely to exist per se. The 8-coordinated dihydrate Ho(C5H7O2)3(H2O)2 is a more plausible formula based on the behavior of other lanthanide acetylacetonates. The dihydrate has been characterized by X-ray crystallography.
Erbium compounds are compounds containing the element erbium (Er). These compounds are usually dominated by erbium in the +3 oxidation state, although the +2, +1 and 0 oxidation states have also been reported.

Yttrium acetylacetonate is a coordination compound with the chemical formula Y(C5H7O2)3(H2O)x, or Y(acac)3(H2O)x for short. The value of x can vary from 1 to 3.

Praseodymium acetylacetonate is a coordination complex with the formula Pr(C3H7O2)3. This purported anhydrous acetylacetonate complex is widely discussed but only the dihydrate Pr(C3H7O2)3(H2O)2 has been characterized by X-ray crystallography.

Erbium acetylacetonate is a coordination compound with the formula Er(C5H7O2)3. This anhydrous acetylacetonate complex is often discussed but unlikely to exist per se. The 8-coordinated dihydrate Er(C5H7O2)3(H2O)2 is a more plausible formula based on the behavior of other lanthanide acetylacetonates. The dihydrate has been characterized by X-ray crystallography.

Lanthanum acetylacetonate refers to the coordination complex with the formula La(C5H7O2)3. This anhydrous acetylacetonate complex has not been characterized well, but the dihydrate La(C5H7O2)3(H2O)2 has been characterized by X-ray crystallography.

Samarium acetylacetonate is a coordination compound with the formula Sm(C5H7O2)3. This anhydrous acetylacetonate complex is widely discussed but unlikely to exist per se. The 8-coordinated dihydrate Sm(C5H7O2)3(H2O)2 is a more plausible formula based on the behavior of other lanthanide acetylacetonates. The dihydrate has been characterized by X-ray crystallography. Upon attempted dehydration by heating under vacuum, other hydrated lanthanide tris(acetylacetonate) complexes decompose to give oxo-clusters.

Lutetium acetylacetonate is a coordination compound with the chemical formula Lu(C5H7O2)3, or Lu(acac)3 for short. The complex per se is unlikely to exist, but the dihydrate would be expected based on the behavior of other lanthanide tris(acetylacetonate)s. Consistent with this scenario, It forms adducts Lu(acac)3(phen) and Lu(acac)3(dipy) where phen and bipy are 1,10-phenanthroline and 2,2'-bipyridine, respectively.

Terbium acetylacetonate is a coordination compound with the formula Tb(C5H7O2)3. This anhydrous acetylacetonate complex is often discussed but unlikely to exist per se. The 8-coordinated dihydrate Tb(C5H7O2)3(H2O)2 is a more plausible formula based on the behavior of other lanthanide acetylacetonates. The dihydrate has been characterized by X-ray crystallography. Upon attempted dehydration by heating under vacuum, other hydrated lanthanide tris(acetylacetonate) complexes decompose to give oxo-clusters. The complex can be prepared from terbium salts, acetylacetone, and a base such as ammonia.


















