Diborane(6), commonly known as diborane, is the chemical compound with the formula B2H6. It is a toxic, colorless, and pyrophoric gas with a repulsively sweet odor. Given its simple formula, borane is a fundamental boron compound. It has attracted wide attention for its electronic structure. Several of its derivatives are useful reagents.

Titanium diboride (TiB2) is an extremely hard ceramic which has excellent heat conductivity, oxidation stability and wear resistance. TiB2 is also a reasonable electrical conductor, so it can be used as a cathode material in aluminium smelting and can be shaped by electrical discharge machining.

Sodium borohydride, also known as sodium tetrahydridoborate and sodium tetrahydroborate, is an inorganic compound with the formula NaBH4. It is a white crystalline solid, usually encountered as an aqueous basic solution. Sodium borohydride is a reducing agent that finds application in papermaking and dye industries. It is also used as a reagent in organic synthesis.

Nickel(II) chloride (or just nickel chloride) is the chemical compound NiCl2. The anhydrous salt is yellow, but the more familiar hydrate NiCl2·6H2O is green. Nickel(II) chloride, in various forms, is the most important source of nickel for chemical synthesis. The nickel chlorides are deliquescent, absorbing moisture from the air to form a solution. Nickel salts have been shown to be carcinogenic to the lungs and nasal passages in cases of long-term inhalation exposure.

Trimethyl borate is the organoboron compound with the formula B(OCH3)3. It is a colourless liquid that burns with a green flame. It is an intermediate in the preparation of sodium borohydride and is a popular reagent in organic chemistry. It is a weak Lewis acid (AN = 23, Gutmann-Beckett method).

Borazine, also known as borazole, is an inorganic compound with the chemical formula B3H6N3. In this cyclic compound, the three BH units and three NH units alternate. The compound is isoelectronic and isostructural with benzene. For this reason borazine is sometimes referred to as “inorganic benzene”. Like benzene, borazine is a colourless liquid with an aromatic odor.

Electroless nickel-phosphorus plating, also referred to as E-nickel, is a chemical process that deposits an even layer of nickel-phosphorus alloy on the surface of a solid substrate, like metal or plastic. The process involves dipping the substrate in a water solution containing nickel salt and a phosphorus-containing reducing agent, usually a hypophosphite salt. It is the most common version of electroless nickel plating and is often referred by that name. A similar process uses a borohydride reducing agent, yielding a nickel-boron coating instead.
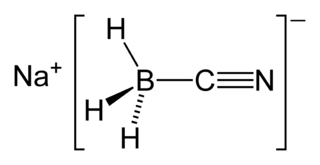
Sodium cyanoborohydride is the chemical compound with the formula Na[BH3(CN)]. It is a colourless salt, but commercial samples can appear tan. It is widely used in organic synthesis for the reduction of imines. The salt tolerates aqueous conditions.

Borohydride refers to the anion [BH4]−, which is also called tetrahydroborate, and its salts. Borohydride or hydroborate is also the term used for compounds containing [BH4−nXn]−, where n is an integer from 0 to 3, for example cyanoborohydride or cyanotrihydroborate [BH3(CN)]− and triethylborohydride or triethylhydroborate [BH(CH2CH3)3]−. Borohydrides find wide use as reducing agents in organic synthesis. The most important borohydrides are lithium borohydride and sodium borohydride, but other salts are well known. Tetrahydroborates are also of academic and industrial interest in inorganic chemistry.
Tetrahydroxyborate is an inorganic anion with the chemical formula [BH4O4]− or [B(OH)4]−. It contributes no colour to tetrahydroxyborate salts. It is found in the mineral hexahydroborite, Ca(B(OH)4)2 · 2 H2O, originally formulated CaB2O4 · 6 H2O. It is one of the boron oxoanions, and acts as a weak base. The systematic names are tetrahydroxyboranuide (substitutive) and tetrahydroxidoborate(1−) (additive). It can be viewed as the conjugate base of boric acid.

Caesium dodecaborate is an inorganic compound with the formula Cs2B12H12. It is a salt composed of caesium and dodecaborate(12) ions. The [B12H12]2− anion has been of great theoretical interest to the chemistry community.
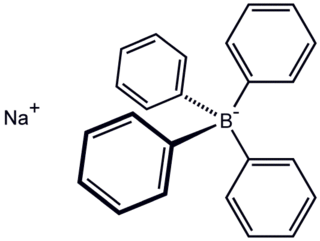
Sodium tetraphenylborate is the organic compound with the formula NaB(C6H5)4. It is a salt, wherein the anion consists of four phenyl rings bonded to boron. This white crystalline solid is used to prepare other tetraphenylborate salts, which are often highly soluble in organic solvents. The compound is used in inorganic and organometallic chemistry as a precipitating agent for potassium, ammonium, rubidium, and cesium ions, and some organic nitrogen compounds.
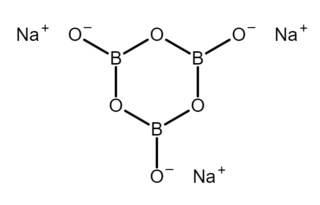
Sodium metaborate is a chemical compound of sodium, boron, and oxygen with formula NaBO2. However, the metaborate ion is trimeric in the anhydrous solid, therefore a more correct formula is Na3B3O6 or (Na+)3[B3O6]3−. The formula can be written also as Na2O·B2O3 to highlight the relation to the main oxides of sodium and boron. The name is also applied to several hydrates whose formulas can be written NaBO2·nH2O for various values of n.

Tetrakis[3,5-bis(trifluoromethyl)phenyl]borate is an anion with chemical formula [{3,5-(CF3)2C6H3}4B]−, which is commonly abbreviated as [BArF4]−, indicating the presence of fluorinated aryl (ArF) groups. It is sometimes referred to as Kobayashi's anion in honour of Hiroshi Kobayashi who led the team that first synthesised it. More commonly it is affectionately nicknamed "BARF." The BARF ion is also abbreviated BArF24−, to distinguish it from the closely related BArF−
20, [(C6F5)4B]−.
Nickel boride is the common name of materials composed chiefly of the elements nickel and boron that are widely used as catalysts in organic chemistry. Their approximate chemical composition is Ni2.5B, and they are often incorrectly denoted "Ni
2B" in organic chemistry publications.

Electroless nickel-boron coating is a metal plating process that can create a layer of a nickel-boron alloy on the surface of a solid substrate, like metal or plastic. The process involves dipping the substrate in a water solution containing nickel salt and a boron-containing reducing agent, such as an alkylamineborane or sodium borohydride. It is a type of electroless nickel plating. A similar process, that uses a hypophosphite as a reducing agent, yields a nickel-phosphorus coating instead.

Iron boride refers to various inorganic compounds with the formula FexBy. Two main iron borides are FeB and Fe2B. Some iron borides possess useful properties such as magnetism, electrical conductivity, corrosion resistance and extreme hardness. Some iron borides have found use as hardening coatings for iron. Iron borides have properties of ceramics such as high hardness, and properties of metal properties, such as thermal conductivity and electrical conductivity. Boride coatings on iron are superior mechanical, frictional, and anti-corrosive. Iron monoboride (FeB) is a grey powder that is insoluble in water. FeB is harder than Fe2B, but is more brittle and more easily fractured upon impact.
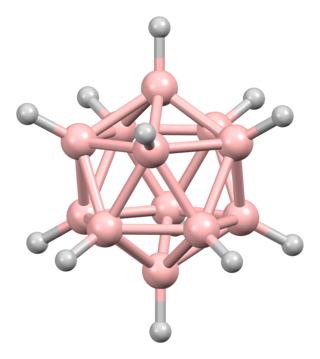
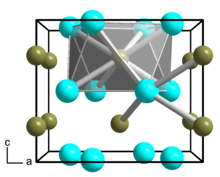
The dodecaborate(12) anion, [B12H12]2−, is a borane with an icosahedral arrangement of 12 boron atoms, with each boron atom being attached to a hydrogen atom. Its symmetry is classified by the molecular point group Ih.
Nickel compounds are chemical compounds containing the element nickel which is a member of the group 10 of the periodic table. Most compounds in the group have an oxidation state of +2. Nickel is classified as a transition metal with nickel(II) having much chemical behaviour in common with iron(II) and cobalt(II). Many salts of nickel(II) are isomorphous with salts of magnesium due to the ionic radii of the cations being almost the same. Nickel forms many coordination complexes. Nickel tetracarbonyl was the first pure metal carbonyl produced, and is unusual in its volatility. Metalloproteins containing nickel are found in biological systems.
Trinickel boride is a compound of nickel and boron with chemical formula Ni
3B. It is one of the borides of nickel.