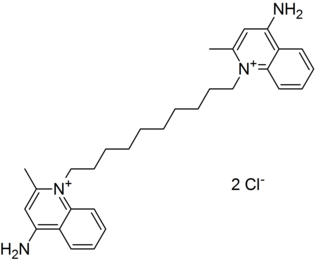A bactericide or bacteriocide, sometimes abbreviated Bcidal, is a substance which kills bacteria. Bactericides are disinfectants, antiseptics, or antibiotics. However, material surfaces can also have bactericidal properties based solely on their physical surface structure, as for example biomaterials like insect wings.

Surfactants are chemical compounds that decrease the surface tension or interfacial tension between two liquids, a liquid and a gas, or a liquid and a solid. The word "surfactant" is a blend of surface-active agent, coined c. 1950. As they consist of a water-repellent and a water-attracting part, they enable water and oil to mix; they can form foam and facilitate the detachment of dirt.

Antibacterial soap is a soap which contains chemical ingredients that purportedly assist in killing bacteria. The majority of antibacterial soaps contain triclosan, though other chemical additives are also common. The effectiveness of products branded as being antibacterial has been disputed by some academics as well as the U.S. Food and Drug Administration (FDA).

A disinfectant is a chemical substance or compound used to inactivate or destroy microorganisms on inert surfaces. Disinfection does not necessarily kill all microorganisms, especially resistant bacterial spores; it is less effective than sterilization, which is an extreme physical or chemical process that kills all types of life. Disinfectants are generally distinguished from other antimicrobial agents such as antibiotics, which destroy microorganisms within the body, and antiseptics, which destroy microorganisms on living tissue. Disinfectants are also different from biocides—the latter are intended to destroy all forms of life, not just microorganisms. Disinfectants work by destroying the cell wall of microbes or interfering with their metabolism. It is also a form of decontamination, and can be defined as the process whereby physical or chemical methods are used to reduce the amount of pathogenic microorganisms on a surface.

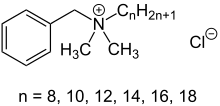
In organic chemistry, quaternary ammonium cations, also known as quats, are positively-charged polyatomic ions of the structure [NR4]+, where R is an alkyl group, an aryl group or organyl group. Unlike the ammonium ion and the primary, secondary, or tertiary ammonium cations, the quaternary ammonium cations are permanently charged, independent of the pH of their solution. Quaternary ammonium salts or quaternary ammonium compounds are salts of quaternary ammonium cations. Polyquats are a variety of engineered polymer forms which provide multiple quat molecules within a larger molecule.
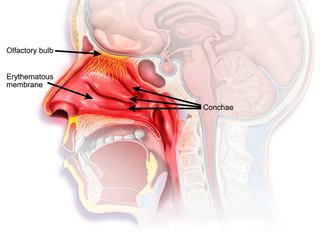
Rhinitis medicamentosa is a condition of rebound nasal congestion suspected to be brought on by extended use of topical decongestants and certain oral medications that constrict blood vessels in the lining of the nose, although evidence has been contradictory.

Hand sanitizer is a liquid, gel or foam generally used to kill many viruses/bacteria/microorganisms on the hands. It can also come in the form of a cream, spray, or wipe. In most settings, hand washing with soap and water is generally preferred. Hand sanitizer is less effective at killing certain kinds of germs, such as norovirus and Clostridium difficile, and unlike hand washing, it cannot physically remove harmful chemicals. People may incorrectly wipe off hand sanitizer before it has dried, and some are less effective because their alcohol concentrations are too low.

Benzethonium chloride, also known as hyamine is a synthetic quaternary ammonium salt. This compound is an odorless white solid, soluble in water. It has surfactant, antiseptic, and anti-infective properties, and it is used as a topical antimicrobial agent in first aid antiseptics. It is also found in cosmetics and toiletries such as soap, mouthwashes, anti-itch ointments, and antibacterial moist towelettes. Benzethonium chloride is also used in the food industry as a hard surface disinfectant.

Lysol is a brand of American cleaning and disinfecting products distributed by Reckitt, which markets the similar Dettol or Sagrotan in other markets. The line includes liquid solutions for hard and soft surfaces, air treatment, and hand washing. The active ingredient in many Lysol products is benzalkonium chloride, but the active ingredient in the Lysol "Power and Free" line is hydrogen peroxide. Lysol has been used since its invention in the late 19th century as a household and industrial cleaning agent, and previously as a medical disinfectant.

Polyaminopropyl biguanide (PAPB) is a disinfectant and a preservative used for disinfection on skin and in cleaning solutions for contact lenses. It is also an ingredient in many deodorant bodysprays. It is a polymer or oligomer where biguanide functional groups are connected by propyl hydrocarbon chains. PAPB is specifically bactericidal at very low concentrations (10 mg/L) and is also fungicidal.
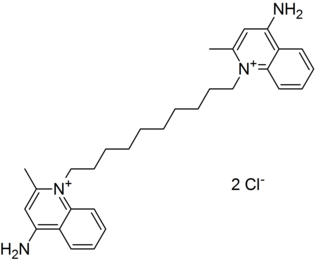
Dequalinium is a quaternary ammonium cation and bolaamphiphile commonly available as the dichloride salt. It is useful as an antiseptic and disinfectant. The bromide, iodide, acetate, and undecenoate salts are known as well. Dequalinium chloride is the active ingredient of several medications.

Cleaning agents or hard-surface cleaners are substances used to remove dirt, including dust, stains, foul odors, and clutter on surfaces. Purposes of cleaning agents include health, beauty, removing offensive odor, and avoiding the spread of dirt and contaminants to oneself and others. Some cleaning agents can kill bacteria and clean at the same time. Others, called degreasers, contain organic solvents to help dissolve oils and fats.
Stearalkonium chloride is a type of benzalkonium chloride which is used as an anti-static agent, a surfactant and an antimicrobial. It is an ingredient in some cosmetics and hair care products, particularly conditioners. It was originally designed by the fabric industry for use as a fabric softener.
A virucide is any physical or chemical agent that deactivates or destroys viruses. The substances are not only virucidal but can be also bactericidal, fungicidal, sporicidal or tuberculocidal.

Cetalkonium chloride (CKC) is a quaternary ammonium compound of the alkyl-benzyldimethylammonium chloride family, the alkyl group having a chain length of C16 . It is used in pharmaceutical products either as an excipient or as an active ingredient. It may be found in very small amount in the excipient benzalkonium chloride mixture. Cetalkonium chloride is purchased as a raw material in dry form as a white powder.
Alkaline copper quaternary, usually abbreviated ACQ, is a type of water-based wood preservative product containing a soluble copper(II) complex and quaternary ammonium alkyl- or aryl-substituted compounds ("quats"). Thus the product was originally called ammoniacal copper/quaternary ammonium.
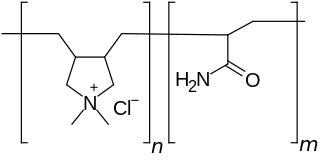
Polyquaternium-7 is an organic compound in the polyquaternium class of chemicals and used in the personal care industry. It is the copolymer of acrylamide and the quaternary ammonium salt diallyldimethylammonium chloride.

Ethyl lauroyl arginate (LAE) is a food preservative, antimicrobial compound, and drug more commonly known as E243. It is used to treat dermatological disorders and is often provided in the form of its hydrochloride salt. LAE is an amino acid-based surfactant with broad-spectrum antimicrobial activity, high biodegradability and low toxicity. Due to these features, LAE is a preservative used in food and cosmetic formulations.
Penetration enhancers are chemical compounds that can facilitate the penetration of active pharmaceutical ingredients (API) into or through the poorly permeable biological membranes. These compounds are used in some pharmaceutical formulations to enhance the penetration of APIs in transdermal drug delivery and transmucosal drug delivery. They typically penetrate into the biological membranes and reversibly decrease their barrier properties.