Related Research Articles

A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium CH+
3, methanium CH+
5 and vinyl C
2H+
3 cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encountered.
In organic chemistry, a carbanion is an anion in which carbon is trivalent and bears a formal negative charge.

In organic chemistry, Hückel's rule predicts that a planar ring molecule will have aromatic properties if it has 4n + 2 π electrons, where n is a non-negative integer. The quantum mechanical basis for its formulation was first worked out by physical chemist Erich Hückel in 1931. The succinct expression as the 4n + 2 rule has been attributed to W. v. E. Doering (1951), although several authors were using this form at around the same time.
Antiaromaticity is a characteristic of a cyclic molecule with a π electron system that has higher energy due to the presence of 4n delocalised electrons in it. Unlike aromatic compounds, which follow Hückel's rule and are highly stable, antiaromatic compounds are highly unstable and highly reactive. To avoid the instability of antiaromaticity, molecules may change shape, becoming non-planar and therefore breaking some of the π interactions. In contrast to the diamagnetic ring current present in aromatic compounds, antiaromatic compounds have a paramagnetic ring current, which can be observed by NMR spectroscopy.
A 1,2-rearrangement or 1,2-migration or 1,2-shift or Whitmore 1,2-shift is an organic reaction where a substituent moves from one atom to another atom in a chemical compound. In a 1,2 shift the movement involves two adjacent atoms but moves over larger distances are possible. In the example below the substituent R moves from carbon atom C2 to C3.

A nucleophilic aromatic substitution is a substitution reaction in organic chemistry in which the nucleophile displaces a good leaving group, such as a halide, on an aromatic ring. Aromatic rings are usually nucleophilic, but some aromatic compounds do undergo nucleophilic substitution. Just as normally nucleophilic alkenes can be made to undergo conjugate substitution if they carry electron-withdrawing substituents, so normally nucleophilic aromatic rings also become electrophilic if they have the right substituents.
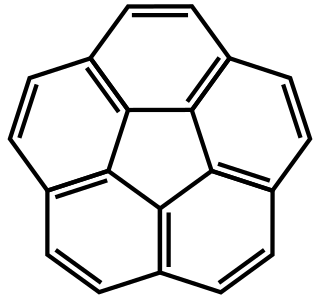
Corannulene is a polycyclic aromatic hydrocarbon with chemical formula C20H10. The molecule consists of a cyclopentane ring fused with 5 benzene rings, so another name for it is [5]circulene. It is of scientific interest because it is a geodesic polyarene and can be considered a fragment of buckminsterfullerene. Due to this connection and also its bowl shape, corannulene is also known as a buckybowl. Buckybowls are fragments of buckyballs. Corannulene exhibits a bowl-to-bowl inversion with an inversion barrier of 10.2 kcal/mol (42.7 kJ/mol) at −64 °C.
A Wagner–Meerwein rearrangement is a class of carbocation 1,2-rearrangement reactions in which a hydrogen, alkyl or aryl group migrates from one carbon to a neighboring carbon. They can be described as cationic [1,2]-sigmatropic rearrangements, proceeding suprafacially and with stereochemical retention. As such, a Wagner–Meerwein shift is a thermally allowed pericyclic process with the Woodward-Hoffmann symbol [ω0s + σ2s]. They are usually facile, and in many cases, they can take place at temperatures as low as –120 °C. The reaction is named after the Russian chemist Yegor Yegorovich Vagner; he had German origin and published in German journals as Georg Wagner; and Hans Meerwein.
In organic chemistry, the term 2-norbornyl cation describes one of the three carbocations formed from derivatives of norbornane. Though 1-norbornyl and 7-norbornyl cations have been studied, the most extensive studies and vigorous debates have been centered on the exact structure of the 2-norbornyl cation.

Homoaromaticity, in organic chemistry, refers to a special case of aromaticity in which conjugation is interrupted by a single sp3 hybridized carbon atom. Although this sp3 center disrupts the continuous overlap of p-orbitals, traditionally thought to be a requirement for aromaticity, considerable thermodynamic stability and many of the spectroscopic, magnetic, and chemical properties associated with aromatic compounds are still observed for such compounds. This formal discontinuity is apparently bridged by p-orbital overlap, maintaining a contiguous cycle of π electrons that is responsible for this preserved chemical stability.
The Gabriel–Colman rearrangement is the chemical reaction of a saccharin or phthalimido ester with a strong base, such as an alkoxide, to form substituted isoquinolines. First described in 1900 by chemists Siegmund Gabriel and James Colman, this rearrangement, a ring expansion, is seen to be general if there is an enolizable hydrogen on the group attached to the nitrogen, since it is necessary for the nitrogen to abstract a hydrogen to form the carbanion that will close the ring. As shown in the case of the general example below, X is either CO or SO2.

Dewar benzene (also spelled dewarbenzene) or bicyclo[2.2.0]hexa-2,5-diene is a bicyclic isomer of benzene with the molecular formula C6H6. The compound is named after James Dewar who included this structure in a list of possible C6H6 structures in 1869. However, he did not propose it as the structure of benzene, and in fact he supported the correct structure previously proposed by August Kekulé in 1865.

Maurice S. Brookhart is an American chemist, and professor of chemistry at the University of Houston since 2015.

A polyquinane polycyclic compound consisting of fused five-membered hydrocarbon rings. If the compound is unsaturated instead of saturated, it is called a polyquinene. The simplest polyquinane is the bicyclic compound bicyclo[3.3.0]octane. Other members are triquinacene and dodecahedrane.
The Stieglitz rearrangement is a rearrangement reaction in organic chemistry which is named after the American chemist Julius Stieglitz (1867–1937) and was first investigated by him and Paul Nicholas Leech in 1913. It describes the 1,2-rearrangement of trityl amine derivatives to triaryl imines. It is comparable to a Beckmann rearrangement which also involves a substitution at a nitrogen atom through a carbon to nitrogen shift. As an example, triaryl hydroxylamines can undergo a Stieglitz rearrangement by dehydration and the shift of a phenyl group after activation with phosphorus pentachloride to yield the respective triaryl imine, a Schiff base.
Saul Winstein was a Jewish Canadian chemist who discovered the Winstein reaction. He argued a non-classical cation was needed to explain the stability of the norbornyl cation. This fueled a debate with Herbert C. Brown over the existence of σ-delocalized carbocations. Winstein also first proposed the concept of an intimate ion pair. He was co-author of the Grunwald–Winstein equation, concerning solvolysis rates.
In chemistry, π-effects or π-interactions are a type of non-covalent interaction that involves π systems. Just like in an electrostatic interaction where a region of negative charge interacts with a positive charge, the electron-rich π system can interact with a metal, an anion, another molecule and even another π system. Non-covalent interactions involving π systems are pivotal to biological events such as protein-ligand recognition.
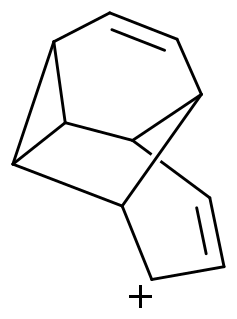
Armilenium is a C
11H+
11 carbocation and was originally proposed as the first entirely organic sandwich compound. Named for its resemblance to an armillary sphere, NMR evidence for the carbocation was first described by Melvin J. Goldstein and Stanley A. Klein at Cornell University in 1973. In subsequent 13C NMR experiments by Goldstein and Joseph P. Dinnocenzo in 1984, the C
11H+
11 carbocation was generated under stable ion conditions at lower temperature and at higher magnetic field than previously possible. These experiments revealed the carbocation to be fluxional. Fitting of the dynamic NMR process ruled out the sandwich species even as an intermediate in the 20-fold degenerate rearrangement of the carbocation.

The Birch reduction is an organic reaction that is used to convert arenes to cyclohexadienes. The reaction is named after the Australian chemist Arthur Birch and involves the organic reduction of aromatic rings in an amine solvent with an alkali metal and a proton source. Unlike catalytic hydrogenation, Birch reduction does not reduce the aromatic ring all the way to a cyclohexane.
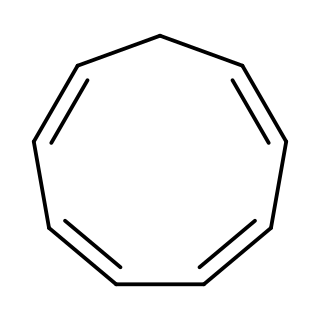
Cyclononatetraene is an organic compound with the formula C9H10. It was first prepared in 1969 by protonation of the corresponding aromatic anion (described below). It is unstable and isomerizes with a half-life of 50 minutes at room temperature to 3a,7a-dihydro-1H-indene via a thermal 6π disrotatory electrocyclic ring closing. Upon exposure to ultraviolet light, it undergoes a photochemical 8π electrocyclic ring closing to give bicyclo[6.1.0]nona-2,4,6-triene.
References
- 1 2 Bicyclic Baird-type aromaticity Won-Young Cha,Taeyeon Kim, Arindam Ghosh,Zhan Zhang,Xian-Sheng Ke, Rashid Ali,Vincent M. Lynch,Jieun Jung, Woojae Kim,Sangsu Lee,Shunichi Fukuzumi,Jung Su Park, Jonathan L. Sessler, Tavarekere K. Chandrashekar & Dongho Kim Nature Chemistry (2017) doi:10.1038/nchem.2834
- ↑ Bicycloaromaticity. 4m + 2, 4n rule Melvin J. Goldstein Journal of the American Chemical Society 1967 89 (24), 6357-6359 doi:10.1021/ja01000a069
- ↑ Rearrangements of bicyclo[3.2.2]nonatrienes Melvin J. Goldstein and B. G. Odell Journal of the American Chemical Society 1967 89 (24), 6356-6357 doi:10.1021/ja01000a068
- ↑ Degenerate Carbocation Rearrangements P.Ahlberg. G.Jonäll , C.Engdahl Advances in Physical Organic Chemistry Volume 19, Pages iii-v, 1-456 (1983) Edited by V. Gold and D. Bethell doi:10.1016/S0065-3160(08)60224-5
- ↑ Bicycloaromaticity. Stability and rearrangement of the bicyclo[3.2.2]nonatrienyl cation John B. Grutzner and Saul Winstein Journal of the American Chemical Society 1970 92 (10), 3186-3187 doi:10.1021/ja00713a045
- ↑ Bicycloaromaticity. Stability and rearrangements of the bicyclo[3.2.2]nonatrienyl anion and cation John B. Grutzner and S. Winstein Journal of the American Chemical Society 1972 94 (7), 2200-2208 doi:10.1021/ja00762a008
- ↑ Homoaromaticity and bicycloaromaticity in carbanions John B. Grutzner and William L. Jorgensen Journal of the American Chemical Society 1981 103 (6), 1372-1375 doi:10.1021/ja00396a013
- ↑ Unusual bicyclic molecule extends frontier of aromaticity Katrina Krämer Chemistry World 2017 link