
Glyphosate is a broad-spectrum systemic herbicide and crop desiccant. It is an organophosphorus compound, specifically a phosphonate, which acts by inhibiting the plant enzyme 5-enolpyruvylshikimate-3-phosphate synthase (EPSP). It is used to kill weeds, especially annual broadleaf weeds and grasses that compete with crops. Its herbicidal effectiveness was discovered by Monsanto chemist John E. Franz in 1970. Monsanto brought it to market for agricultural use in 1974 under the trade name Roundup. Monsanto's last commercially relevant United States patent expired in 2000.

Bifenthrin is a pyrethroid insecticide. It is widely used against ant infestations.

Dicofol is an insecticide, an organochlorine that is chemically related to DDT. Dicofol is a miticide that is very effective against spider mite. Its production and use is banned internationally under the Stockholm Convention.

Alachlor is an herbicide from the chloroacetanilide family. It is an odorless, white solid. The greatest use of alachlor is for control of annual grasses and broadleaf weeds in crops. Use of alachlor is illegal in the European Union and no products containing alachlor are currently registered in the United States.
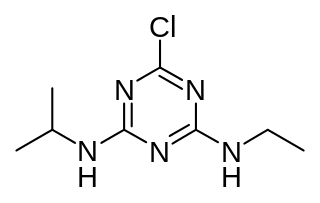
Atrazine is a chlorinated herbicide of the triazine class. It is used to prevent pre-emergence broadleaf weeds in crops such as maize (corn), soybean and sugarcane and on turf, such as golf courses and residential lawns. Atrazine's primary manufacturer is Syngenta and it is one of the most widely used herbicides in the United States, Canadian, and Australian agriculture. Its use was banned in the European Union in 2004, when the EU found groundwater levels exceeding the limits set by regulators, and Syngenta could not show that this could be prevented nor that these levels were safe.
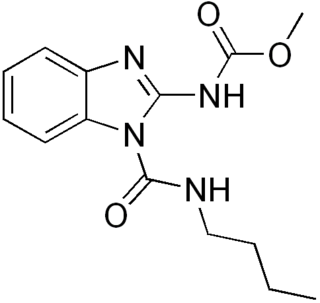
Benomyl is a fungicide introduced in 1968 by DuPont. It is a systemic benzimidazole fungicide that is selectively toxic to microorganisms and invertebrates, but relatively nontoxic toward mammals.
Iodomethane, also called methyl iodide, and commonly abbreviated "MeI", is the chemical compound with the formula CH3I. It is a dense, colorless, volatile liquid. In terms of chemical structure, it is related to methane by replacement of one hydrogen atom by an atom of iodine. It is naturally emitted by rice plantations in small amounts. It is also produced in vast quantities estimated to be greater than 214,000 tons annually by algae and kelp in the world's temperate oceans, and in lesser amounts on land by terrestrial fungi and bacteria. It is used in organic synthesis as a source of methyl groups.

Endrin is an organochlorine compound with the chemical formula C12H8Cl6O that was first produced in 1950 by Shell and Velsicol Chemical Corporation. It was primarily used as an insecticide, as well as a rodenticide and piscicide. It is a colourless, odorless solid, although commercial samples are often off-white. Endrin was manufactured as an emulsifiable solution known commercially as Endrex. The compound became infamous as a persistent organic pollutant and for this reason it is banned in many countries.

Soil contamination, soil pollution, or land pollution as a part of land degradation is caused by the presence of xenobiotic (human-made) chemicals or other alteration in the natural soil environment. It is typically caused by industrial activity, agricultural chemicals or improper disposal of waste. The most common chemicals involved are petroleum hydrocarbons, polynuclear aromatic hydrocarbons, solvents, pesticides, lead, and other heavy metals. Contamination is correlated with the degree of industrialization and intensity of chemical substance. The concern over soil contamination stems primarily from health risks, from direct contact with the contaminated soil, vapour from the contaminants, or from secondary contamination of water supplies within and underlying the soil. Mapping of contaminated soil sites and the resulting clean ups are time-consuming and expensive tasks, and require expertise in geology, hydrology, chemistry, computer modelling, and GIS in Environmental Contamination, as well as an appreciation of the history of industrial chemistry.

Methoxychlor is a synthetic organochloride insecticide, now obsolete. Tradenames for methoxychlor include Chemform, Maralate, Methoxo, Methoxcide, Metox, and Moxie.

Dimethyl phthalate (DMP) is an organic compound and phthalate ester. it is a colourless and oily liquid that is soluble in organic solvents, but which is only poorly soluble in water.

Dinoseb is a common industry name for 6-sec-butyl-2,4-dinitrophenol, a herbicide in the dinitrophenol family. It is a crystalline orange solid which does not readily dissolve in water. Dinoseb is banned as an herbicide in the European Union (EU) and the United States because of its toxicity.
Drinking water quality in the United States is generally safe. In 2016, over 90 percent of the nation's community water systems were in compliance with all published U.S. Environmental Protection Agency standards. Over 286 million Americans get their tap water from a community water system. Eight percent of the community water systems—large municipal water systems—provide water to 82 percent of the US population. The Safe Drinking Water Act requires the US EPA to set standards for drinking water quality in public water systems. Enforcement of the standards is mostly carried out by state health agencies. States may set standards that are more stringent than the federal standards.

1,2,3-Trichloropropane (TCP) is an organic compound with the formula CHCl(CH2Cl)2. It is a colorless liquid that is used as a solvent and in other specialty applications.

2,4-Dichlorophenoxyacetic acid is an organic compound with the chemical formula Cl2C6H3OCH2CO2H. It is usually referred to by its ISO common name 2,4-D. It is a systemic herbicide that kills most broadleaf weeds by causing uncontrolled growth, but most grasses such as cereals, lawn turf, and grassland are relatively unaffected.

Imazaquin is an imidazolinone herbicide, so named because it contains an imidazolinone core. This organic compound is used to control a broad spectrum of weed species. It is a colorless or white solid, although commercial samples can appear brown or tan.

Bentazon is a chemical manufactured by BASF Chemicals for use in herbicides. It is categorized under the thiadiazine group of chemicals. Sodium bentazon is available commercially and appears slightly brown in colour.
Glyphosate-based herbicides are usually made of a glyphosate salt that is combined with other ingredients that are needed to stabilize the herbicide formula and allow penetration into plants. The glyphosate-based herbicide Roundup was first developed by Monsanto in the 1970s. It is used most heavily on corn, soy, and cotton crops that have been genetically modified to be resistant to the herbicide. Some products include two active ingredients, such as Enlist Duo which includes 2,4-D as well as glyphosate. As of 2010, more than 750 glyphosate products were on the market. The names of inert ingredients used in glyphosate formulations are usually not listed on the product labels.

Cyanazine is a herbicide that belongs to the group of triazines. Cyanazine inhibits photosynthesis and is therefore used as a herbicide.

Ohio River Park is a Superfund Site located in Neville Island, Pennsylvania. Between the 1920s-1970s, the Site was used for municipal waste, pesticide manufacturing, coke sludge disposal, cement manufacturing disposal, and pesticide waste. In 1977, Neville Land Company donated the Site to Allegheny County who started developing the Site as a community park. In 1979, Allegheny County found various hazardous contaminants on the Site. On August 30, 1990, the Site was determined to be a Superfund Site due to VOCs, SVOCs, inorganics, and pesticides being present in the surface soil, subsurface soil, surface water, river sediment, and groundwater. Soil remediation began in February 1998 and ended in September 1999. Today, Ohio River Park has the Robert Morris University Island Sports Center and Coraopolis Bridge on top of it. Additionally, benzene continues to be monitored because it is still present in the Site's groundwater.




















