
The flash point of a material is the "lowest liquid temperature at which, under certain standardized conditions, a liquid gives off vapours in a quantity such as to be capable of forming an ignitable vapour/air mixture".
Coal dust is a fine-powdered form of coal which is created by the crushing, grinding, or pulverization of coal rock. Because of the brittle nature of coal, coal dust can be created by mining, transporting, or mechanically handling it.
The autoignition temperature or self-ignition temperature, often called spontaneous ignition temperature or minimum ignition temperature and formerly also known as kindling point, of a substance is the lowest temperature in which it spontaneously ignites in a normal atmosphere without an external source of ignition, such as a flame or spark. This temperature is required to supply the activation energy needed for combustion. The temperature at which a chemical ignites decreases as the pressure is increased.
Decane is an alkane hydrocarbon with the chemical formula C10H22. Although 75 structural isomers are possible for decane, the term usually refers to the normal-decane ("n-decane"), with the formula CH3(CH2)8CH3. All isomers, however, exhibit similar properties and little attention is paid to the composition. These isomers are flammable liquids. Decane is present in small quantities (less than 1%) in gasoline (petrol) and kerosene. Like other alkanes, it is a nonpolar solvent, and does not dissolve in water, and is readily combustible. Although it is a component of fuels, it is of little importance as a chemical feedstock, unlike a handful of other alkanes.

A backdraft or backdraught is the abrupt burning of superheated gases in a fire caused when oxygen rapidly enters a hot, oxygen-depleted environment; for example, when a window or door to an enclosed space is opened or broken. Backdrafts are typically seen as a blast of smoke and/or flame out of an opening of a building. Backdrafts present a serious threat to firefighters. There is some debate concerning whether backdrafts should be considered a type of flashover.
A flashover is the near-simultaneous ignition of most of the directly exposed combustible material in an enclosed area. When certain organic materials are heated, they undergo thermal decomposition and release flammable gases. Flashover occurs when the majority of the exposed surfaces in a space are heated to their autoignition temperature and emit flammable gases. Flashover normally occurs at 500 °C (932 °F) or 590 °C (1,100 °F) for ordinary combustibles and an incident heat flux at floor level of 20 kilowatts per square metre (2.5 hp/sq ft).

The fire triangle or combustion triangle is a simple model for understanding the necessary ingredients for most fires.
A flash fire is a sudden, intense fire caused by ignition of a mixture of air and a dispersed flammable substance such as a solid, flammable or combustible liquid, or a flammable gas. It is characterized by high temperature, short duration, and a rapidly moving flame front.

In electrical and safety engineering, hazardous locations are places where fire or explosion hazards may exist. Sources of such hazards include gases, vapors, dust, fibers, and flyings, which are combustible or flammable. Electrical equipment installed in such locations can provide an ignition source, due to electrical arcing, or high temperatures. Standards and regulations exist to identify such locations, classify the hazards, and design equipment for safe use in such locations.
A fire class is a system of categorizing fire with regard to the type of material and fuel for combustion. Class letters are often assigned to the different types of fire, but these differ between territories; there are separate standards for the United States, Europe, and Australia. The fire class is used to determine the types of extinguishing agents that can be used for that category.

A combustible material is a material that can burn in air under certain conditions. A material is flammable if it ignites easily at ambient temperatures. In other words, a combustible material ignites with some effort and a flammable material catches fire immediately on exposure to flame.

A dust explosion is the rapid combustion of fine particles suspended in the air within an enclosed location. Dust explosions can occur where any dispersed powdered combustible material is present in high-enough concentrations in the atmosphere or other oxidizing gaseous medium, such as pure oxygen. In cases when fuel plays the role of a combustible material, the explosion is known as a fuel-air explosion.
A gas detector is a device that detects the presence of gases in an area, often as part of a safety system. A gas detector can sound an alarm to operators in the area where the leak is occurring, giving them the opportunity to leave. This type of device is important because there are many gases that can be harmful to organic life, such as humans or animals.
A flammable liquid is a liquid with flash point of not more than 60.5 °C (141 °F), or any material in a liquid phase with a flash point at or above 37.8 °C (100 °F) that is intentionally heated and offered for transportation or transported at or above its flash point in a bulk packaging.
The lower flammability limit (LFL), usually expressed in volume per cent, is the lower end of the concentration range over which a flammable mixture of gas or vapour in air can be ignited at a given temperature and pressure. The flammability range is delineated by the upper and lower flammability limits. Outside this range of air/vapor mixtures, the mixture cannot be ignited at that temperature and pressure. The LFL decreases with increasing temperature; thus, a mixture that is below its LFL at a given temperature may be ignitable if heated sufficiently.
An infrared point sensor is a point gas detector based on the nondispersive infrared sensor technology.
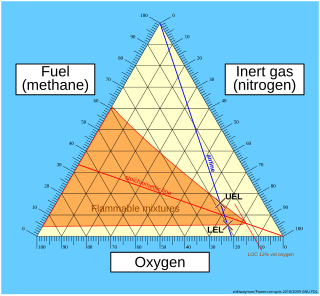
Flammability diagrams show the control of flammability in mixtures of fuel, oxygen and an inert gas, typically nitrogen. Mixtures of the three gasses are usually depicted in a triangular diagram, known as a ternary plot. Such diagrams are available in the speciality literature. The same information can be depicted in a normal orthogonal diagram, showing only two substances, implicitly using the feature that the sum of all three components is 100 percent. The diagrams below only concerns one fuel; the diagrams can be generalized to mixtures of fuels.
In fire and explosion prevention engineering, purging refers to the introduction of an inert purge gas into a closed system to prevent the formation of an ignitable atmosphere. Purging relies on the principle that a combustible gas is able to undergo combustion (explode) only if mixed with air in the right proportions. The flammability limits of the gas define those proportions, i.e. the ignitable range.
In fire and explosion prevention engineering, inerting refers to the introduction of an inert (non-combustible) gas into a closed system to make a flammable atmosphere oxygen deficient and non-ignitable.

A high pressure jet is a stream of pressurized fluid that is released from an environment at a significantly higher pressure than ambient pressure from a nozzle or orifice, due to operational or accidental release. In the field of safety engineering, the release of toxic and flammable gases has been the subject of many R&D studies because of the major risk that they pose to the health and safety of workers, equipment and environment. Intentional or accidental release may occur in an industrial settings like natural gas processing plants, oil refineries and hydrogen storage facilities.










