
Brassicaceae or Cruciferae is a medium-sized and economically important family of flowering plants commonly known as the mustards, the crucifers, or the cabbage family. Most are herbaceous plants, while some are shrubs. The leaves are simple, lack stipules, and appear alternately on stems or in rosettes. The inflorescences are terminal and lack bracts. The flowers have four free sepals, four free alternating petals, two shorter free stamens and four longer free stamens. The fruit has seeds in rows, divided by a thin wall.

In organic chemistry, isothiocyanate is the functional group −N=C=S, formed by substituting the oxygen in the isocyanate group with a sulfur. Many natural isothiocyanates from plants are produced by enzymatic conversion of metabolites called glucosinolates. These natural isothiocyanates, such as allyl isothiocyanate, are also known as mustard oils. An artificial isothiocyanate, phenyl isothiocyanate, is used for amino acid sequencing in the Edman degradation.
A glucoside is a glycoside that is chemically derived from glucose. Glucosides are common in plants, but rare in animals. Glucose is produced when a glucoside is hydrolysed by purely chemical means, or decomposed by fermentation or enzymes.

Mustard oil can mean either the pressed oil used for cooking, or a pungent essential oil also known as volatile oil of mustard. The essential oil results from grinding mustard seed, mixing the grounds with water, and isolating the resulting volatile oil by distillation. It can also be produced by dry distillation of the seed. Pressed mustard oil is used as cooking oil in some cultures, but sale is restricted in some countries due to high levels of erucic acid. Varieties of mustard seed also exist that are low in erucic acid.

Allyl isothiocyanate (AITC) is a naturally occurring unsaturated isothiocyanate. The colorless oil is responsible for the pungent taste of Cruciferous vegetables such as mustard, radish, horseradish, and wasabi. This pungency and the lachrymatory effect of AITC are mediated through the TRPA1 and TRPV1 ion channels. It is slightly soluble in water, but more soluble in most organic solvents.

Glucoraphanin is a glucosinolate found in broccoli, mustard and other cruciferous vegetables.

Glucosinolates are natural components of many pungent plants such as mustard, cabbage, and horseradish. The pungency of those plants is due to mustard oils produced from glucosinolates when the plant material is chewed, cut, or otherwise damaged. These natural chemicals most likely contribute to plant defence against pests and diseases, and impart a characteristic bitter flavor property to cruciferous vegetables.
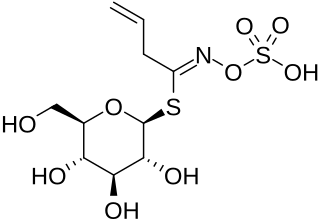
Sinigrin or allyl glucosinolate is a glucosinolate that belongs to the family of glucosides found in some plants of the family Brassicaceae such as Brussels sprouts, broccoli, and the seeds of black mustard. Whenever sinigrin-containing plant tissue is crushed or otherwise damaged, the enzyme myrosinase degrades sinigrin to a mustard oil, which is responsible for the pungent taste of mustard and horseradish. Seeds of white mustard, Sinapis alba, give a less pungent mustard because this species contains a different glucosinolate, sinalbin.

Glucobrassicin is a type of glucosinolate that can be found in almost all cruciferous plants, such as cabbages, broccoli, mustards, and woad. As for other glucosinolates, degradation by the enzyme myrosinase is expected to produce an isothiocyanate, indol-3-ylmethylisothiocyanate. However, this specific isothiocyanate is expected to be highly unstable, and has indeed never been detected. The observed hydrolysis products when isolated glucobrassicin is degraded by myrosinase are indole-3-carbinol and thiocyanate ion, which are envisioned to result from a rapid reaction of the unstable isothiocyanate with water. However, a large number of other reaction products are known, and indole-3-carbinol is not the dominant degradation product when glucosinolate degradation takes place in crushed plant tissue or in intact plants.

Myrosinase is a family of enzymes involved in plant defense against herbivores, specifically the mustard oil bomb. The three-dimensional structure has been elucidated and is available in the PDB.

Erysimum cheiranthoides, the treacle-mustard,wormseed wallflower, or wormseed mustard is a species of Erysimum native to most of central and northern Europe and northern and central Asia. Like other Erysimum species, E. cheiranthoides accumulates two major classes of defensive chemicals: glucosinolates and cardiac glycosides.
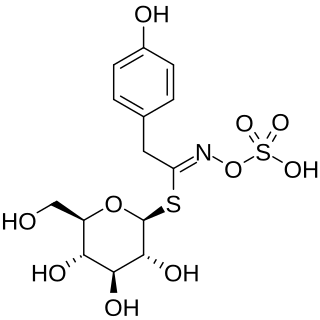
Sinalbin is a glucosinolate found in the seeds of white mustard, Sinapis alba, and in many wild plant species. In contrast to mustard from black mustard seeds which contain sinigrin, mustard from white mustard seeds has only a weakly pungent taste.

Gluconasturtiin or phenethyl glucosinolate is one of the most widely distributed glucosinolates in the cruciferous vegetables, mainly in the roots, and is probably one of the plant compounds responsible for the natural pest-inhibiting properties of growing crucifers, such as cabbage, mustard or rape, in rotation with other crops. This effect of gluconasturtiin is due to its degradation by the plant enzyme myrosinase into phenethyl isothiocyanate, which is toxic to many organisms.

Brevicoryne brassicae, commonly known as the cabbage aphid or cabbage aphis, is a destructive aphid native to Europe that is now found in many other areas of the world. The aphids feed on many varieties of produce, including cabbage, broccoli (especially), Brussels sprouts, cauliflower and many other members of the genus Brassica, but do not feed on plants outside of the family Brassicaceae. The insects entirely avoid plants other than those of Brassicaceae; even though thousands may be eating broccoli near strawberries, the strawberries will be left untouched.
In enzymology, a N-hydroxythioamide S-beta-glucosyltransferase is an enzyme that catalyzes the chemical reaction

Reseda luteola is a flowering plant species in the family Resedaceae. Common names include dyer's rocket, dyer's weed, weld, woold, and yellow weed. A native of Europe and Western Asia, the plant can be found in North America as an introduced species and common weed. While other resedas were used for the purpose, this species was the most widely used source of the natural dye known as weld. The plant is rich in luteolin, a flavonoid which produces a bright yellow dye. The yellow could be mixed with the blue from woad to produce greens such as Lincoln green.

(R)-prunasin is a cyanogenic glycoside related to amygdalin. Chemically, it is the glucoside of (R)-mandelonitrile.
Phenylalanine N-monooxygenase (EC 1.14.14.40, phenylalanine N-hydroxylase, CYP79A2) is an enzyme with systematic name L-phenylalanine,NADPH:oxygen oxidoreductase (N-hydroxylating). This enzyme catalyses the following chemical reaction
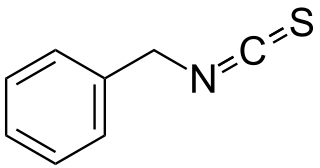
Benzyl isothiocyanate (BITC) is an isothiocyanate found in plants of the mustard family.

















