
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6H5, and is often represented by the symbol Ph. The phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen, which may be replaced by some other element or compound to serve as a functional group. A phenyl group has six carbon atoms bonded together in a hexagonal planar ring, five of which are bonded to individual hydrogen atoms, with the remaining carbon bonded to a substituent. Phenyl groups are commonplace in organic chemistry. Although often depicted with alternating double and single bonds, the phenyl group is chemically aromatic and has equal bond lengths between carbon atoms in the ring.

Aniline is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starting material for fine chemical synthesis. Its main use is in the manufacture of precursors to polyurethane, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish. It ignites readily, burning with a smoky flame characteristic of aromatic compounds. It is toxic to humans.

Quinoline is a heterocyclic aromatic organic compound with the chemical formula C9H7N. It is a colorless hygroscopic liquid with a strong odor. Aged samples, especially if exposed to light, become yellow and later brown. Quinoline is only slightly soluble in cold water but dissolves readily in hot water and most organic solvents. Quinoline itself has few applications, but many of its derivatives are useful in diverse applications. A prominent example is quinine, an alkaloid found in plants. Over 200 biologically active quinoline and quinazoline alkaloids are identified. 4-Hydroxy-2-alkylquinolines (HAQs) are involved in antibiotic resistance.

In organic chemistry, isothiocyanate is the functional group −N=C=S, formed by substituting the oxygen in the isocyanate group with a sulfur. Many natural isothiocyanates from plants are produced by enzymatic conversion of metabolites called glucosinolates. These natural isothiocyanates, such as allyl isothiocyanate, are also known as mustard oils. An artificial isothiocyanate, phenyl isothiocyanate, is used for amino acid sequencing in the Edman degradation.
In organic chemistry, an aryl halide is an aromatic compound in which one or more hydrogen atoms, directly bonded to an aromatic ring are replaced by a halide. The haloarene are different from haloalkanes because they exhibit many differences in methods of preparation and properties. The most important members are the aryl chlorides, but the class of compounds is so broad that there are many derivatives and applications.
Edman degradation, developed by Pehr Edman, is a method of sequencing amino acids in a peptide. In this method, the amino-terminal residue is labeled and cleaved from the peptide without disrupting the peptide bonds between other amino acid residues.
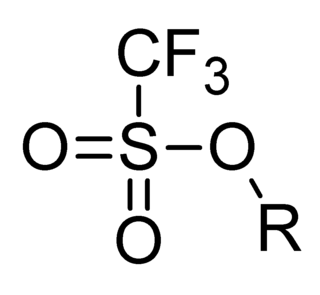
In organic chemistry, triflate, is a functional group with the formula R−OSO2CF3 and structure R−O−S(=O)2−CF3. The triflate group is often represented by −OTf, as opposed to −Tf, which is the triflyl group, R−SO2CF3. For example, n-butyl triflate can be written as CH3CH2CH2CH2OTf.
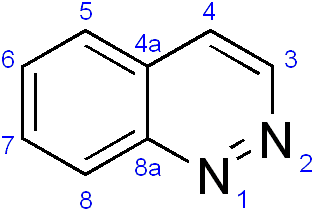
Cinnoline is an aromatic heterocyclic compound with the formula C8H6N2. It is isomeric with other naphthyridines including quinoxaline, phthalazine and quinazoline.

Thiophenol is an organosulfur compound with the formula C6H5SH, sometimes abbreviated as PhSH. This foul-smelling colorless liquid is the simplest aromatic thiol. The chemical structures of thiophenol and its derivatives are analogous to phenols. An exception is the oxygen atom in the hydroxyl group (-OH) bonded to the aromatic ring is replaced by a sulfur atom. The prefix thio- implies a sulfur-containing compound and when used before a root word name for a compound which would normally contain an oxygen atom, in the case of 'thiol' that the alcohol oxygen atom is replaced by a sulfur atom.
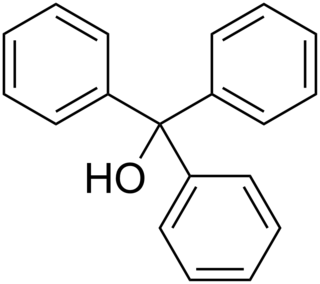
Triphenylmethanol is an organic compound. It is a white crystalline solid that is insoluble in water and petroleum ether, but well soluble in ethanol, diethyl ether, and benzene. In strongly acidic solutions, it produces an intensely yellow color, due to the formation of a stable "trityl" carbocation. Many derivatives of triphenylmethanol are important dyes.

Iodobenzene is an organoiodine compound consisting of a benzene ring substituted with one iodine atom. It is useful as a synthetic intermediate in organic chemistry. It is a volatile colorless liquid, although aged samples appear yellowish.

Thiocarbanilide is an organic chemical compound with the formula (C6H5NH)2CS. This white solid is a derivative of thiourea. It is prepared by the reaction of aniline and carbon disulfide.

Organomercury chemistry refers to the study of organometallic compounds that contain mercury. Typically the Hg–C bond is stable toward air and moisture but sensitive to light. Important organomercury compounds are the methylmercury(II) cation, CH3Hg+; ethylmercury(II) cation, C2H5Hg+; dimethylmercury, (CH3)2Hg, diethylmercury and merbromin ("Mercurochrome"). Thiomersal is used as a preservative for vaccines and intravenous drugs.
Phenylphosphine is an organophosphorus compound with the chemical formula C6H5PH2. It is the phosphorus analog of aniline. Like other primary phosphines, phenylphosphine has an intense penetrating odor and is highly oxidizable. It is mainly used as a precursor to other organophosphorus compounds. It can function as a ligand in coordination chemistry.

The Gould–Jacobs reaction is an organic synthesis for the preparation of quinolines and 4‐hydroxyquinoline derivatives. The Gould–Jacobs reaction is a series of reactions. The series of reactions begins with the condensation/substitution of an aniline with alkoxy methylenemalonic ester or acyl malonic ester, producing anilidomethylenemalonic ester. Then through a 6 electron cyclization process, 4-hydroxy-3-carboalkoxyquinoline is formed, which exist mostly in the 4-oxo form. Saponification results in the formation of an acid. This step is followed by decarboxylation to give 4-hydroxyquinoline. The Gould–Jacobs reaction is effective for anilines with electron‐donating groups at the meta‐position.
Trifluorotoluene is an organic compound with the formula of C6H5CF3. This colorless fluorocarbon is used as a specialty solvent in organic synthesis and an intermediate in the production of pesticides and pharmaceuticals.

Benzenediazonium tetrafluoroborate is an organic compound with the formula [C6H5N2]BF4. It is a salt of a diazonium cation and tetrafluoroborate. It exists as a colourless solid that is soluble in polar solvents. It is the parent member of the aryldiazonium compounds, which are widely used in organic chemistry.

Organobismuth chemistry is the chemistry of organometallic compounds containing a carbon to bismuth chemical bond. Applications are few. The main bismuth oxidation states are Bi(III) and Bi(V) as in all higher group 15 elements. The energy of a bond to carbon in this group decreases in the order P > As > Sb > Bi. The first reported use of bismuth in organic chemistry was in oxidation of alcohols by Frederick Challenger in 1934 (using Ph3Bi(OH)2). Knowledge about methylated species of bismuth in environmental and biological media is limited.
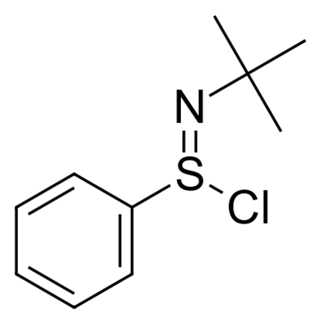
N-tert-Butylbenzenesulfinimidoyl chloride is a useful oxidant for organic synthesis reactions. It is a good electrophile, and the sulfimide S=N bond can be attacked by nucleophiles, such as alkoxides, enolates, and amide ions. The nitrogen atom in the resulting intermediate is basic, and can abstract an α-hydrogen to create a new double bond.
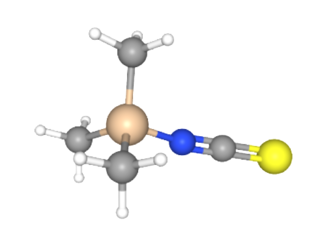
Trimethylsilyl isothiocyanate (TMSNCS) is an organosilicon compound that contains an isothiocyanate whose nitrogen atom is covalently bonded to a trimethylsilyl group. The isothiocyanate group is an analog of the isocyanate group, but having a sulfur instead of oxygen.


 [3]
[3] 

















