
In optics, the refractive index of an optical medium is a dimensionless number that gives the indication of the light bending ability of that medium.

Birefringence is the optical property of a material having a refractive index that depends on the polarization and propagation direction of light. These optically anisotropic materials are described as birefringent or birefractive. The birefringence is often quantified as the maximum difference between refractive indices exhibited by the material. Crystals with non-cubic crystal structures are often birefringent, as are plastics under mechanical stress.

Polydiacetylenes (PDAs) are a family of conducting polymers closely related to polyacetylene. They are created by the 1,4 topochemical polymerization of diacetylenes. They have multiple applications from the development of organic films to immobilization of other molecules.

In organic chemistry, an imide is a functional group consisting of two acyl groups bound to nitrogen. The compounds are structurally related to acid anhydrides, although imides are more resistant to hydrolysis. In terms of commercial applications, imides are best known as components of high-strength polymers, called polyimides. Inorganic imides are also known as solid state or gaseous compounds, and the imido group (=NH) can also act as a ligand.

Polyimide is a polymer containing imide groups belonging to the class of high-performance plastics. With their high heat-resistance, polyimides enjoy diverse applications in roles demanding rugged organic materials, such as high temperature fuel cells, displays, and various military roles. A classic polyimide is Kapton, which is produced by condensation of pyromellitic dianhydride and 4,4'-oxydianiline.

A dielectric mirror, also known as a Bragg mirror, is a type of mirror composed of multiple thin layers of dielectric material, typically deposited on a substrate of glass or some other optical material. By careful choice of the type and thickness of the dielectric layers, one can design an optical coating with specified reflectivity at different wavelengths of light. Dielectric mirrors are also used to produce ultra-high reflectivity mirrors: values of 99.999% or better over a narrow range of wavelengths can be produced using special techniques. Alternatively, they can be made to reflect a broad spectrum of light, such as the entire visible range or the spectrum of the Ti-sapphire laser.
Ormosil is a shorthand phrase for organically modified silica or organically modified silicate. In general, ormosils are produced by adding silane to silica-derived gel during the sol-gel process. They are engineered materials that show great promise in a wide range of applications such as:

Nanocomposite is a multiphase solid material where one of the phases has one, two or three dimensions of less than 100 nanometers (nm) or structures having nano-scale repeat distances between the different phases that make up the material.

Heptacene is an organic compound and a polycyclic aromatic hydrocarbon and the seventh member of the acene or polyacene family of linear fused benzene rings. This compound has long been pursued by chemists because of its potential interest in electronic applications and was first synthesized but not cleanly isolated in 2006. Heptacene was finally fully characterized in bulk by researchers in Germany and the United States in 2017.
Polymer nanocomposites (PNC) consist of a polymer or copolymer having nanoparticles or nanofillers dispersed in the polymer matrix. These may be of different shape, but at least one dimension must be in the range of 1–50 nm. These PNC's belong to the category of multi-phase systems that consume nearly 95% of plastics production. These systems require controlled mixing/compounding, stabilization of the achieved dispersion, orientation of the dispersed phase, and the compounding strategies for all MPS, including PNC, are similar. Alternatively, polymer can be infiltrated into 1D, 2D, 3D preform creating high content polymer nanocomposites.

A silsesquioxane is an organosilicon compound with the chemical formula [RSiO3/2]n. Silsesquioxanes are colorless solids that adopt cage-like or polymeric structures with Si-O-Si linkages and tetrahedral Si vertices. Silsesquioxanes are members of polyoctahedral silsesquioxanes ("POSS"), which have attracted attention as preceramic polymer precursors to ceramic materials and nanocomposites. Diverse substituents (R) can be attached to the Si centers. The molecules are unusual because they feature an inorganic silicate core and an organic exterior. The silica core confers rigidity and thermal stability.
A thermoset polymer matrix is a synthetic polymer reinforcement where polymers act as binder or matrix to secure in place incorporated particulates, fibres or other reinforcements. They were first developed for structural applications, such as glass-reinforced plastic radar domes on aircraft and graphite-epoxy payload bay doors on the Space Shuttle.
Fire-safe polymers are polymers that are resistant to degradation at high temperatures. There is need for fire-resistant polymers in the construction of small, enclosed spaces such as skyscrapers, boats, and airplane cabins. In these tight spaces, ability to escape in the event of a fire is compromised, increasing fire risk. In fact, some studies report that about 20% of victims of airplane crashes are killed not by the crash itself but by ensuing fires. Fire-safe polymers also find application as adhesives in aerospace materials, insulation for electronics, and in military materials such as canvas tenting.

The Birch reduction is an organic reaction that is used to convert arenes to 1,4-cyclohexadienes. The reaction is named after the Australian chemist Arthur Birch and involves the organic reduction of aromatic rings in an amine solvent with an alkali metal and a proton source. Unlike catalytic hydrogenation, Birch reduction does not reduce the aromatic ring all the way to a cyclohexane.
Polymers with the ability to kill or inhibit the growth of microorganisms such as bacteria, fungi, or viruses are classified as antimicrobial agents. This class of polymers consists of natural polymers with inherent antimicrobial activity and polymers modified to exhibit antimicrobial activity. Polymers are generally nonvolatile, chemically stable, and can be chemically and physically modified to display desired characteristics and antimicrobial activity. Antimicrobial polymers are a prime candidate for use in the food industry to prevent bacterial contamination and in water sanitation to inhibit the growth of microorganisms in drinking water.

Polyfluorene is a polymer with formula (C13H8)n, consisting of fluorene units linked in a linear chain — specifically, at carbon atoms 2 and 7 in the standard fluorene numbering. It can also be described as a chain of benzene rings linked in para positions with an extra methylene bridge connecting every pair of rings.
Oxycarbide glass, also referred to as silicon oxycarbide, is a type of glass that contains oxygen and carbon in addition to silicon dioxide. It is created by substituting some oxygen atoms with carbon atoms. This glass may contain particles of amorphous carbon, and silicon carbide. SiOC materials of varying stoichiometery are attractive owing to their generally high density, hardness and high service temperatures. Through diverse forming techniques high performance parts in complex shapes can be achieved. Unlike pure SiC, the versatile stoichiometry of SiOC offers further avenues to tune physical properties through appropriate selection of processing parameters.
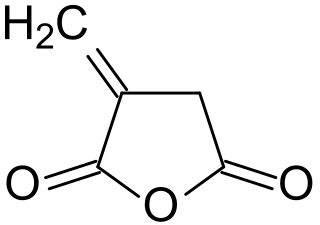
Itaconic anhydride is the cyclic anhydride of itaconic acid and is obtained by the pyrolysis of citric acid. It is a colourless, crystalline solid, which dissolves in many polar organic solvents and hydrolyzes forming itaconic acid. Itaconic anhydride and its derivative itaconic acid have been promoted as biobased "platform chemicals" and bio- building blocks.) These expectations, however, have not been fulfilled.

β-Butyrolactone is the intramolecular carboxylic acid ester (lactone) of the optically active 3-hydroxybutanoic acid. It is produced during chemical synthesis as a racemate. β-Butyrolactone is suitable as a monomer for the production of the biodegradable polyhydroxyalkanoate poly(3-hydroxybutyrate) (PHB). Polymerisation of racemic (RS)-β-butyrolactone provides (RS)-polyhydroxybutyric acid, which, however, is inferior in essential properties (e.g. strength or degradation behaviour) to the (R)-poly-3-hydroxybutyrate originating from natural sources.
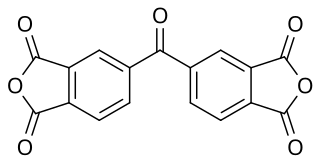
3,3’,4,4’-Benzophenone tetracarboxylic dianhydride (BTDA) is chemically, an aromatic organic acid dianhydride. It may be used to cure epoxy-based powder coatings. It has the CAS Registry Number of 2421-28-5 and a European Community number 219-348-1. It is REACH and TSCA registered. The formula is C17H6O7 with a molecular weight of 322.3.





























