A DNA vaccine is a type of vaccine that transfects a specific antigen-coding DNA sequence into the cells of an organism as a mechanism to induce an immune response.

Macrophages are a type of white blood cell of the innate immune system that engulf and digest pathogens, such as cancer cells, microbes, cellular debris, and foreign substances, which do not have proteins that are specific to healthy body cells on their surface. This process is called phagocytosis, which acts to defend the host against infection and injury.

The T helper cells (Th cells), also known as CD4+ cells or CD4-positive cells, are a type of T cell that play an important role in the adaptive immune system. They aid the activity of other immune cells by releasing cytokines. They are considered essential in B cell antibody class switching, breaking cross-tolerance in dendritic cells, in the activation and growth of cytotoxic T cells, and in maximizing bactericidal activity of phagocytes such as macrophages and neutrophils. CD4+ cells are mature Th cells that express the surface protein CD4. Genetic variation in regulatory elements expressed by CD4+ cells determines susceptibility to a broad class of autoimmune diseases.
Immunoglobulin E (IgE) is a type of antibody that has been found only in mammals. IgE is synthesised by plasma cells. Monomers of IgE consist of two heavy chains and two light chains, with the ε chain containing four Ig-like constant domains (Cε1–Cε4). IgE is thought to be an important part of the immune response against infection by certain parasitic worms, including Schistosoma mansoni, Trichinella spiralis, and Fasciola hepatica. IgE is also utilized during immune defense against certain protozoan parasites such as Plasmodium falciparum. IgE may have evolved as a defense to protect against venoms.

Cellular immunity, also known as cell-mediated immunity, is an immune response that does not rely on the production of antibodies. Rather, cell-mediated immunity is the activation of phagocytes, antigen-specific cytotoxic T-lymphocytes, and the release of various cytokines in response to an antigen.

The interleukin 4 is a cytokine that induces differentiation of naive helper T cells (Th0 cells) to Th2 cells. Upon activation by IL-4, Th2 cells subsequently produce additional IL-4 in a positive feedback loop. IL-4 is produced primarily by mast cells, Th2 cells, eosinophils and basophils. It is closely related and has functions similar to IL-13.
Gut-associated lymphoid tissue (GALT) is a component of the mucosa-associated lymphoid tissue (MALT) which works in the immune system to protect the body from invasion in the gut.
Interleukin 13 (IL-13) is a protein that in humans is encoded by the IL13 gene. IL-13 was first cloned in 1993 and is located on chromosome 5q31.1 with a length of 1.4kb. It has a mass of 13 kDa and folds into 4 alpha helical bundles. The secondary structural features of IL-13 are similar to that of Interleukin 4 (IL-4); however it only has 25% sequence identity to IL-4 and is capable of IL-4 independent signaling. IL-13 is a cytokine secreted by T helper type 2 (Th2) cells, CD4 cells, natural killer T cell, mast cells, basophils, eosinophils and nuocytes. Interleukin-13 is a central regulator in IgE synthesis, goblet cell hyperplasia, mucus hypersecretion, airway hyperresponsiveness, fibrosis and chitinase up-regulation. It is a mediator of allergic inflammation and different diseases including asthma.
Memory T cells are a subset of T lymphocytes that might have some of the same functions as memory B cells. Their lineage is unclear.
Immune dysregulation is any proposed or confirmed breakdown or maladaptive change in molecular control of immune system processes. For example, dysregulation is a component in the pathogenesis of autoimmune diseases and some cancers. Immune system dysfunction, as seen in IPEX syndrome leads to immune dysfunction, polyendocrinopathy, enteropathy, X-linked (IPEX). IPEX typically presents during the first few months of life with diabetes mellitus, intractable diarrhea, failure to thrive, eczema, and hemolytic anemia. unrestrained or unregulated immune response.
Interleukin 33 (IL-33) is a protein that in humans is encoded by the IL33 gene.

Interleukin-25 (IL-25) – also known as interleukin-17E (IL-17E) – is a protein that in humans is encoded by the IL25 gene on chromosome 14. IL-25 was discovered in 2001 and is made up of 177 amino acids.
Interleukin-22 receptor subunit alpha-2 (IL-22RA2), also known as interleukin-22 binding protein (IL-22BP) is a naturally secreted monomeric protein acting as an interleukin-22 (IL-22) antagonist with inhibitory effects on IL-22 activity in vivo. IL-22BP is in humans encoded by the IL22RA2 gene located on chromosome 6, and in mice is encoded by the il22ra2 gene located on chromosome 10. IL-22BP belongs to the class II cytokine receptor family and it is a soluble receptor homolog of IL-22R.
T helper 17 cells (Th17) are a subset of pro-inflammatory T helper cells defined by their production of interleukin 17 (IL-17). They are related to T regulatory cells and the signals that cause Th17s to actually inhibit Treg differentiation. However, Th17s are developmentally distinct from Th1 and Th2 lineages. Th17 cells play an important role in maintaining mucosal barriers and contributing to pathogen clearance at mucosal surfaces; such protective and non-pathogenic Th17 cells have been termed as Treg17 cells.
Gamma delta T cells are T cells that have a γδ T-cell receptor (TCR) on their surface. Most T cells are αβ T cells with TCR composed of two glycoprotein chains called α (alpha) and β (beta) TCR chains. In contrast, γδ T cells have a TCR that is made up of one γ (gamma) chain and one δ (delta) chain. This group of T cells is usually less common than αβ T cells. Their highest abundance is in the gut mucosa, within a population of lymphocytes known as intraepithelial lymphocytes (IELs).

Mucosal immunology is the study of immune system responses that occur at mucosal membranes of the intestines, the urogenital tract, and the respiratory system. The mucous membranes are in constant contact with microorganisms, food, and inhaled antigens. In healthy states, the mucosal immune system protects the organism against infectious pathogens and maintains a tolerance towards non-harmful commensal microbes and benign environmental substances. Disruption of this balance between tolerance and deprivation of pathogens can lead to pathological conditions such as food allergies, irritable bowel syndrome, susceptibility to infections, and more.
The nuocyte is a cell of the innate immune system that plays an important role in type 2 immune responses that are induced in response to helminth worm infection or in conditions such as asthma and atopic disease. Nuocytes are amongst the first cells activated in type 2 immune responses and are thought to play important roles in activating and recruiting other cells types through their production of type 2 cytokines interleukin 4, 5 and 13. Nuocytes have been observed to proliferate in the presence of interleukin 7 (IL-7) in vitro. Nuocytes contribute to the expulsion of helminth worms and to the pathology of colitis and allergic airways disease.
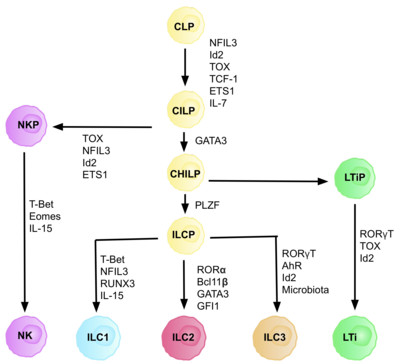
Innate lymphoid cells (ILCs) are the most recently discovered family of innate immune cells, derived from common lymphoid progenitors (CLPs). In response to pathogenic tissue damage, ILCs contribute to immunity via the secretion of signalling molecules, and the regulation of both innate and adaptive immune cells. ILCs are primarily tissue resident cells, found in both lymphoid, and non- lymphoid tissues, and rarely in the blood. They are particularly abundant at mucosal surfaces, playing a key role in mucosal immunity and homeostasis. Characteristics allowing their differentiation from other immune cells include the regular lymphoid morphology, absence of rearranged antigen receptors found on T cells and B cells, and phenotypic markers usually present on myeloid or dendritic cells.

Type 3 innate lymphoid cells (ILC3) are immune cells from the lymphoid lineage that are part of the innate immune system. These cells participate in innate mechanisms on mucous membranes, contributing to tissue homeostasis, host-commensal mutualism and pathogen clearance. They are part of a heterogeneous group of innate lymphoid cells, which is traditionally divided into three subsets based on their expression of master transcription factors as well as secreted effector cytokines - ILC1, ILC2 and ILC3.
Type 2 inflammation is a pattern of immune response. Its physiological function is to defend the body against helminths, but a dysregulation of the type 2 inflammatory response has been implicated in the pathophysiology of several diseases.