
Aluminium is a chemical element; it has symbol Al and atomic number 13. Aluminium has a density lower than that of other common metals; about one-third that of steel. It has a great affinity towards oxygen, forming a protective layer of oxide on the surface when exposed to air. Aluminium visually resembles silver, both in its color and in its great ability to reflect light. It is soft, nonmagnetic and ductile. It has one stable isotope: 27Al, which is highly abundant, making aluminium the twelfth-most common element in the universe. The radioactivity of 26Al is used in radiometric dating.

Bauxite is a sedimentary rock with a relatively high aluminium content. It is the world's main source of aluminium and gallium. Bauxite consists mostly of the aluminium minerals gibbsite (Al(OH)3), boehmite (γ-AlO(OH)) and diaspore (α-AlO(OH)), mixed with the two iron oxides goethite (FeO(OH)) and haematite (Fe2O3), the aluminium clay mineral kaolinite (Al2Si2O5(OH)4) and small amounts of anatase (TiO2) and ilmenite (FeTiO3 or FeO.TiO2). Bauxite appears dull in luster and is reddish-brown, white, or tan.

Corundum is a crystalline form of aluminium oxide typically containing traces of iron, titanium, vanadium, and chromium. It is a rock-forming mineral. It is a naturally transparent material, but can have different colors depending on the presence of transition metal impurities in its crystalline structure. Corundum has two primary gem varieties: ruby and sapphire. Rubies are red due to the presence of chromium, and sapphires exhibit a range of colors depending on what transition metal is present. A rare type of sapphire, padparadscha sapphire, is pink-orange.

Thermite is a pyrotechnic composition of metal powder and metal oxide. When ignited by heat or chemical reaction, thermite undergoes an exothermic reduction-oxidation (redox) reaction. Most varieties are not explosive, but can create brief bursts of heat and high temperature in a small area. Its form of action is similar to that of other fuel-oxidizer mixtures, such as black powder.
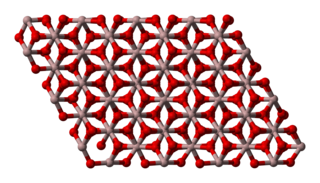
Aluminium oxide (or aluminium(III) oxide) is a chemical compound of aluminium and oxygen with the chemical formula Al2O3. It is the most commonly occurring of several aluminium oxides, and specifically identified as aluminium oxide. It is commonly called alumina and may also be called aloxide, aloxite, or alundum in various forms and applications. It occurs naturally in its crystalline polymorphic phase α-Al2O3 as the mineral corundum, varieties of which form the precious gemstones ruby and sapphire. Al2O3 is significant in its use to produce aluminium metal, as an abrasive owing to its hardness, and as a refractory material owing to its high melting point.

Calcium oxide, commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term lime connotes calcium-containing inorganic compounds, in which carbonates, oxides, and hydroxides of calcium, silicon, magnesium, aluminium, and iron predominate. By contrast, quicklime specifically applies to the single compound calcium oxide. Calcium oxide that survives processing without reacting in building products, such as cement, is called free lime.
The Hall–Héroult process is the major industrial process for smelting aluminium. It involves dissolving aluminium oxide (alumina) in molten cryolite, and electrolyzing the molten salt bath, typically in a purpose-built cell. The Hall–Héroult process applied at industrial scale happens at 940–980 °C and produces 99.5–99.8% pure aluminium. Recycled aluminum requires no electrolysis, thus it does not end up in this process.
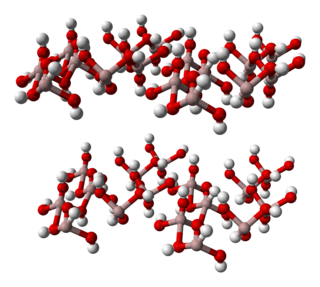
Aluminium hydroxide, Al(OH)3, is found in nature as the mineral gibbsite and its three much rarer polymorphs: bayerite, doyleite, and nordstrandite. Aluminium hydroxide is amphoteric, i.e., it has both basic and acidic properties. Closely related are aluminium oxide hydroxide, AlO(OH), and aluminium oxide or alumina, the latter of which is also amphoteric. These compounds together are the major components of the aluminium ore bauxite. Aluminium hydroxide also forms a gelatinous precipitate in water.
The Bayer process is the principal industrial means of refining bauxite to produce alumina (aluminium oxide) and was developed by Carl Josef Bayer. Bauxite, the most important ore of aluminium, contains only 30–60% aluminium oxide (Al2O3), the rest being a mixture of silica, various iron oxides, and titanium dioxide. The aluminium oxide must be further purified before it can be refined into aluminium metal.

In materials science, a refractory is a material that is resistant to decomposition by heat or chemical attack that retains its strength and rigidity at high temperatures. They are inorganic, non-metallic compounds that may be porous or non-porous, and their crystallinity varies widely: they may be crystalline, polycrystalline, amorphous, or composite. They are typically composed of oxides, carbides or nitrides of the following elements: silicon, aluminium, magnesium, calcium, boron, chromium and zirconium. Many refractories are ceramics, but some such as graphite are not, and some ceramics such as clay pottery are not considered refractory. Refractories are distinguished from the refractory metals, which are elemental metals and their alloys that have high melting temperatures.
Aluminum Corporation of China Limited, is a multinational aluminium company headquartered in Beijing, People's Republic of China. It is a public company, listed in Hong Kong and in New York. In 2021, it was the world's largest aluminum producer, ahead of China Hongqiao Group, Rusal and Shandong Xinfa.

Aluminium recycling is the process in which secondary aluminium is created from scrap or other forms of end-of-life or otherwise unusable aluminium. It involves re-melting the metal, which is cheaper and more energy-efficient than the production of aluminum from raw bauxite via electrolysis of aluminum oxide (Al2O3) during the Hall–Héroult and Bayer processes.

United Company RUSAL, international public joint-stock company is the world's second largest aluminium company by primary production output. It was the largest until overtaken by China Hongqiao Group in 2015. UC RUSAL accounts for almost 9% of the world's primary aluminium output and 9% of the world's alumina production.
Aluminium–air batteries produce electricity from the reaction of oxygen in the air with aluminium. They have one of the highest energy densities of all batteries, but they are not widely used because of problems with high anode cost and byproduct removal when using traditional electrolytes. This has restricted their use to mainly military applications. However, an electric vehicle with aluminium batteries has the potential for up to eight times the range of a lithium-ion battery with a significantly lower total weight.

Volta Aluminum Company, known as VALCO, is an aluminium company based in Tema, Greater Accra Region founded by Kaiser Aluminum and now wholly owned by the government of Ghana.

Aluminium smelting is the process of extracting aluminium from its oxide, alumina, generally by the Hall-Héroult process. Alumina is extracted from the ore bauxite by means of the Bayer process at an alumina refinery.

Red mud, now more frequently termed bauxite residue, is an industrial waste generated during the processing of bauxite into alumina using the Bayer process. It is composed of various oxide compounds, including the iron oxides which give its red colour. Over 95% of the alumina produced globally is through the Bayer process; for every tonne of alumina produced, approximately 1 to 1.5 tonnes of red mud are also produced. Annual production of alumina in 2020 was over 133 million tonnes resulting in the generation of over 175 million tonnes of red mud.

Nanosized aluminium oxide occurs in the form of spherical or nearly spherical nanoparticles, and in the form of oriented or undirected fibers.

China Hongqiao Group Limited is a company founded in 1994 that specializes in the production of aluminium. Hongqiao is currently the second largest aluminium producer in the world after Chinalco. It is listed on the Hong Kong Stock Exchange with stock code 1378, and is incorporated in George Town, Cayman Islands.

Aluminium metal is very rare in native form, and the process to refine it from ores is complex, so for most of human history it was unknown. However, the compound alum has been known since the 5th century BCE and was used extensively by the ancients for dyeing. During the Middle Ages, its use for dyeing made it a commodity of international commerce. Renaissance scientists believed that alum was a salt of a new earth; during the Age of Enlightenment, it was established that this earth, alumina, was an oxide of a new metal. Discovery of this metal was announced in 1825 by Danish physicist Hans Christian Ørsted, whose work was extended by German chemist Friedrich Wöhler.
















