Parkinsonism is a clinical syndrome characterized by tremor, bradykinesia, rigidity, and postural instability. These are the four motor symptoms found in Parkinson's disease (PD) – after which it is named – dementia with Lewy bodies (DLB), Parkinson's disease dementia (PDD), and many other conditions. This set of symptoms occurs in a wide range of conditions and may have many causes, including neurodegenerative conditions, drugs, toxins, metabolic diseases, and neurological conditions other than PD.

Multiple system atrophy (MSA) is a rare neurodegenerative disorder characterized by autonomic dysfunction, tremors, slow movement, muscle rigidity, and postural instability and ataxia. This is caused by progressive degeneration of neurons in several parts of the brain including the basal ganglia, inferior olivary nucleus, and cerebellum.

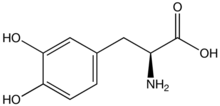
l-DOPA, also known as levodopa and l-3,4-dihydroxyphenylalanine, is made and used as part of the normal biology of some plants and animals, including humans. Humans, as well as a portion of the other animals that utilize l-DOPA, make it via biosynthesis from the amino acid l-tyrosine. l-DOPA is the precursor to the neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline), which are collectively known as catecholamines. Furthermore, l-DOPA itself mediates neurotrophic factor release by the brain and CNS. In some plant families, l-DOPA is the central precursor of a biosynthetic pathway that produces a class of pigments called betalains. l-DOPA can be manufactured and in its pure form is sold as a psychoactive drug with the INN levodopa; trade names include Sinemet, Pharmacopa, Atamet, and Stalevo. As a drug, it is used in the clinical treatment of Parkinson's disease and dopamine-responsive dystonia.
Dyskinesia refers to a category of movement disorders that are characterized by involuntary muscle movements, including movements similar to tics or chorea and diminished voluntary movements. Dyskinesia can be anything from a slight tremor of the hands to an uncontrollable movement of the upper body or lower extremities. Discoordination can also occur internally especially with the respiratory muscles and it often goes unrecognized. Dyskinesia is a symptom of several medical disorders that are distinguished by their underlying cause.

Progressive supranuclear palsy (PSP) is a late-onset neurodegenerative disease involving the gradual deterioration and death of specific volumes of the brain. The condition leads to symptoms including loss of balance, slowing of movement, difficulty moving the eyes, and cognitive impairment. PSP may be mistaken for other types of neurodegeneration such as Parkinson's disease, frontotemporal dementia and Alzheimer's disease. The cause of the condition is uncertain, but involves the accumulation of tau protein within the brain. Medications such as levodopa and amantadine may be useful in some cases.
Hypokinesia is one of the classifications of movement disorders, and refers to decreased bodily movement. Hypokinesia is characterized by a partial or complete loss of muscle movement due to a disruption in the basal ganglia. Hypokinesia is a symptom of Parkinson's disease shown as muscle rigidity and an inability to produce movement. It is also associated with mental health disorders and prolonged inactivity due to illness, amongst other diseases.
Parkinson-plus syndromes (PPS) are a group of neurodegenerative diseases featuring the classical features of Parkinson's disease with additional features that distinguish them from simple idiopathic Parkinson's disease (PD). Parkinson-plus syndromes are either inherited genetically or occur sporadically.

Fall prevention includes any action taken to help reduce the number of accidental falls suffered by susceptible individuals, such as the elderly (idiopathic) and people with neurological or orthopedic indications.
In the management of Parkinson's disease, due to the chronic nature of Parkinson's disease (PD), a broad-based program is needed that includes patient and family education, support-group services, general wellness maintenance, exercise, and nutrition. At present, no cure for the disease is known, but medications or surgery can provide relief from the symptoms.

Droxidopa is a synthetic amino acid precursor which acts as a prodrug to the neurotransmitter norepinephrine (noradrenaline). Unlike norepinephrine, droxidopa is capable of crossing the protective blood–brain barrier (BBB).
Dopamine-responsive dystonia (DRD) also known as Segawa syndrome (SS), is a genetic movement disorder which usually manifests itself during early childhood at around ages 5–8 years.

Dopamine dysregulation syndrome (DDS) is a dysfunction of the reward system observed in some individuals taking dopaminergic medications for an extended length of time. It typically occurs in people with Parkinson's disease (PD) who have taken dopamine agonist medications for an extended period of time. It is characterized by problems such as addiction to medication, gambling, or sexual behavior.

Parkinson's disease (PD), or simply Parkinson's, is a chronic degenerative disorder of the central nervous system that affects both the motor system and non-motor systems. The symptoms usually emerge slowly, and as the disease progresses, non-motor symptoms become more common. Early symptoms are tremor, rigidity, slowness of movement, and difficulty with walking. Problems may also arise with cognition, behaviour, sleep, and sensory systems. Parkinson's disease dementia is common in advanced stages.
Signs and symptoms of Parkinson's disease are varied. Parkinson's disease affects movement, producing motor symptoms. Non-motor symptoms, which include dysautonomia, cognitive and neurobehavioral problems, and sensory and sleep difficulties, are also common. When other diseases mimic Parkinson's disease, they are categorized as parkinsonism.
Levodopa-induced dyskinesia (LID) is a form of dyskinesia associated with levodopa (l-DOPA), used to treat Parkinson's disease. It often involves hyperkinetic movements, including chorea, dystonia, and athetosis.

The history of Parkinson's disease expands from 1817, when British apothecary James Parkinson published An Essay on the Shaking Palsy, to modern times. Before Parkinson's descriptions, others had already described features of the disease that would bear his name, while the 20th century greatly improved knowledge of the disease and its treatments. PD was then known as paralysis agitans. The term "Parkinson's disease" was coined in 1865 by William Sanders and later popularized by French neurologist Jean-Martin Charcot.
Kinesia paradoxa is a phenomenon most often seen in people with Parkinson's disease where individuals who typically experience severe difficulties with the simple movements may perform complex movements easily. Specifically, kinesia paradoxa focuses on walking, referring to the sudden ability to demonstrate smooth, fluid movements in people that previously had problems with walking easily. This new discovery does not just happen to an individual randomly, but must be stimulated using various types of visual or auditory cues.
The temporal dynamics of music and language describes how the brain coordinates its different regions to process musical and vocal sounds. Both music and language feature rhythmic and melodic structure. Both employ a finite set of basic elements that are combined in ordered ways to create complete musical or lingual ideas.
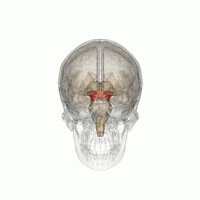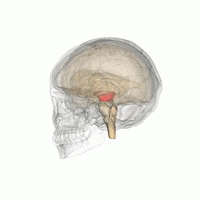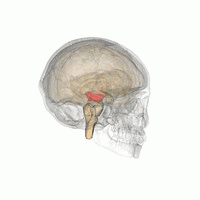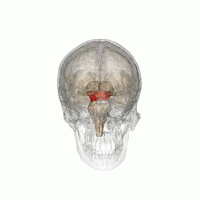
The mesencephalic locomotor region (MLR) is a functionally defined area of the midbrain that is associated with the initiation and control of locomotor movements in vertebrate species.
Gait variability seen in Parkinson's Disorders arise due to cortical changes induced by pathophysiology of the disease process. Gait rehabilitation is focused to harness the adapted connections involved actively to control these variations during the disease progression. Gait variabilities seen are attributed to the defective inputs from the Basal Ganglia. However, there is altered activation of other cortical areas that support the deficient control to bring about a movement and maintain some functional mobility.