Related Research Articles
Antioxidants are compounds that inhibit oxidation, a chemical reaction that can produce free radicals. Autoxidation leads to degradation of organic compounds, including living matter. Antioxidants are frequently added to industrial products, such as polymers, fuels, and lubricants, to extend their usable lifetimes. Food are also treated with antioxidants to forestall spoilage, in particular the rancidification of oils and fats. In cells, antioxidants such as glutathione, mycothiol or bacillithiol, and enzyme systems like superoxide dismutase, can prevent damage from oxidative stress.

Superoxide dismutase (SOD, EC 1.15.1.1) is an enzyme that alternately catalyzes the dismutation (or partitioning) of the superoxide (O−
2) radical into ordinary molecular oxygen (O2) and hydrogen peroxide (H
2O
2). Superoxide is produced as a by-product of oxygen metabolism and, if not regulated, causes many types of cell damage. Hydrogen peroxide is also damaging and is degraded by other enzymes such as catalase. Thus, SOD is an important antioxidant defense in nearly all living cells exposed to oxygen. One exception is Lactobacillus plantarum and related lactobacilli, which use a different mechanism to prevent damage from reactive O−
2.
Glutathione is an antioxidant in plants, animals, fungi, and some bacteria and archaea. Glutathione is capable of preventing damage to important cellular components caused by sources such as reactive oxygen species, free radicals, peroxides, lipid peroxides, and heavy metals. It is a tripeptide with a gamma peptide linkage between the carboxyl group of the glutamate side chain and cysteine. The carboxyl group of the cysteine residue is attached by normal peptide linkage to glycine.
In chemistry, a superoxide is a compound that contains the superoxide ion, which has the chemical formula O−2. The systematic name of the anion is dioxide(1−). The reactive oxygen ion superoxide is particularly important as the product of the one-electron reduction of dioxygen O2, which occurs widely in nature. Molecular oxygen (dioxygen) is a diradical containing two unpaired electrons, and superoxide results from the addition of an electron which fills one of the two degenerate molecular orbitals, leaving a charged ionic species with a single unpaired electron and a net negative charge of −1. Both dioxygen and the superoxide anion are free radicals that exhibit paramagnetism. Superoxide was historically also known as "hyperoxide".
Xanthine oxidase is a form of xanthine oxidoreductase, a type of enzyme that generates reactive oxygen species. These enzymes catalyze the oxidation of hypoxanthine to xanthine and can further catalyze the oxidation of xanthine to uric acid. These enzymes play an important role in the catabolism of purines in some species, including humans.
The free radical theory of aging states that organisms age because cells accumulate free radical damage over time. A free radical is any atom or molecule that has a single unpaired electron in an outer shell. While a few free radicals such as melanin are not chemically reactive, most biologically relevant free radicals are highly reactive. For most biological structures, free radical damage is closely associated with oxidative damage. Antioxidants are reducing agents, and limit oxidative damage to biological structures by passivating them from free radicals.

In chemistry, reactive oxygen species (ROS) are highly reactive chemicals formed from diatomic oxygen. Examples of ROS include peroxides, superoxide, hydroxyl radical, singlet oxygen, and alpha-oxygen.

Paraquat (trivial name; ), or N,N′-dimethyl-4,4′-bipyridinium dichloride (systematic name), also known as methyl viologen, is an organic compound with the chemical formula [(C6H7N)2]Cl2. It is classified as a viologen, a family of redox-active heterocycles of similar structure. This salt is one of the most widely used herbicides. It is quick-acting and non-selective, killing green plant tissue on contact. It is also toxic (lethal) to human beings and animals due to its redox activity, which produces superoxide anions. It has been linked to the development of Parkinson's disease and is banned in several countries.

Lipid peroxidation is the chain of reactions of oxidative degradation of lipids. It is the process in which free radicals "steal" electrons from the lipids in cell membranes, resulting in cell damage. This process proceeds by a free radical chain reaction mechanism. It most often affects polyunsaturated fatty acids, because they contain multiple double bonds in between which lie methylene bridges (-CH2-) that possess especially reactive hydrogen atoms. As with any radical reaction, the reaction consists of three major steps: initiation, propagation, and termination. The chemical products of this oxidation are known as lipid peroxides or lipid oxidation products (LOPs).
Respiratory burst is the rapid release of the reactive oxygen species (ROS), superoxide anion and hydrogen peroxide, from different cell types.
NADPH oxidase is a membrane-bound enzyme complex that faces the extracellular space. It can be found in the plasma membrane as well as in the membranes of phagosomes used by neutrophil white blood cells to engulf microorganisms. Human isoforms of the catalytic component of the complex include NOX1, NOX2, NOX3, NOX4, NOX5, DUOX1, and DUOX2.

Oxidative stress reflects an imbalance between the systemic manifestation of reactive oxygen species and a biological system's ability to readily detoxify the reactive intermediates or to repair the resulting damage. Disturbances in the normal redox state of cells can cause toxic effects through the production of peroxides and free radicals that damage all components of the cell, including proteins, lipids, and DNA. Oxidative stress from oxidative metabolism causes base damage, as well as strand breaks in DNA. Base damage is mostly indirect and caused by the reactive oxygen species generated, e.g., O2− (superoxide radical), OH (hydroxyl radical) and H2O2 (hydrogen peroxide). Further, some reactive oxidative species act as cellular messengers in redox signaling. Thus, oxidative stress can cause disruptions in normal mechanisms of cellular signaling.
Myricetin is a member of the flavonoid class of polyphenolic compounds, with antioxidant properties. Common dietary sources include vegetables, fruits, nuts, berries, tea, and red wine. Myricetin is structurally similar to fisetin, luteolin, and quercetin and is reported to have many of the same functions as these other members of the flavonol class of flavonoids. Reported average intake of myricetin per day varies depending on diet, but has been shown in the Netherlands to average 23 mg/day.
Glutathione synthetase (GSS) is the second enzyme in the glutathione (GSH) biosynthesis pathway. It catalyses the condensation of gamma-glutamylcysteine and glycine, to form glutathione. Glutathione synthetase is also a potent antioxidant. It is found in many species including bacteria, yeast, mammals, and plants.

In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron. With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spontaneously dimerize. Most organic radicals have short lifetimes.

Bacterial glutathione transferases are part of a superfamily of enzymes that play a crucial role in cellular detoxification. The primary role of GSTs is to catalyze the conjugation of glutathione (GSH) with the electrophilic centers of a wide variety of molecules. The most commonly known substrates of GSTs are xenobiotic synthetic chemicals. There are also classes of GSTs that utilize glutathione as a cofactor rather than a substrate. Often these GSTs are involved in reduction of reactive oxidative species toxic to the bacterium. Conjugation with glutathione receptors renders toxic substances more soluble, and therefore more readily exocytosed from the cell.
All living cells produce reactive oxygen species (ROS) as a byproduct of metabolism. ROS are reduced oxygen intermediates that include the superoxide radical (O2−) and the hydroxyl radical (OH•), as well as the non-radical species hydrogen peroxide (H2O2). These ROS are important in the normal functioning of cells, playing a role in signal transduction and the expression of transcription factors. However, when present in excess, ROS can cause damage to proteins, lipids and DNA by reacting with these biomolecules to modify or destroy their intended function. As an example, the occurrence of ROS have been linked to the aging process in humans, as well as several other diseases including Alzheimer's, rheumatoid arthritis, Parkinson's, and some cancers. Their potential for damage also makes reactive oxygen species useful in direct protection from invading pathogens, as a defense response to physical injury, and as a mechanism for stopping the spread of bacteria and viruses by inducing programmed cell death.
Oxidation response is stimulated by a disturbance in the balance between the production of reactive oxygen species and antioxidant responses, known as oxidative stress. Active species of oxygen naturally occur in aerobic cells and have both intracellular and extracellular sources. These species, if not controlled, damage all components of the cell, including proteins, lipids and DNA. Hence cells need to maintain a strong defense against the damage. The following table gives an idea of the antioxidant defense system in bacterial system.
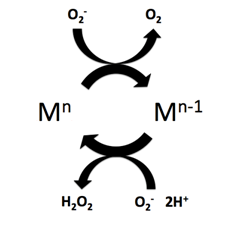
Superoxide dismutase (SOD) mimetics are synthetic compounds that mimic the native superoxide dismutase enzyme. SOD mimetics effectively convert the superoxide anion, a reactive oxygen species, into hydrogen peroxide, which is further converted into water by catalase. Reactive oxygen species are natural byproducts of cellular respiration and cause oxidative stress and cell damage, which has been linked to causing cancers, neurodegeneration, age-related declines in health, and inflammatory diseases. SOD mimetics are a prime interest in therapeutic treatment of oxidative stress because of their smaller size, longer half-life, and similarity in function to the native enzyme.
Antioxidative stress is an overabundance of bioavailable antioxidant compounds that interfere with the immune system's ability to neutralize pathogenic threats. The fundamental opposite is oxidative stress, which can lead to such disease states as coronary heart disease or cancer.
References
- ↑ Puglia CD, Powell SR (1984). "Inhibition of cellular antioxidants: a possible mechanism of toxic cell injury". Environ. Health Perspect. 57: 307–11. doi:10.2307/3429932. JSTOR 3429932. PMC 1568295 . PMID 6094175.
- ↑ James LP, Mayeux PR, Hinson JA (2003). "Acetaminophen-induced hepatotoxicity". Drug Metab. Dispos. 31 (12): 1499–506. doi:10.1124/dmd.31.12.1499. PMID 14625346.
- ↑ Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ (2002). "Mechanisms of hepatotoxicity". Toxicol. Sci. 65 (2): 166–76. doi:10.1093/toxsci/65.2.166. PMID 11812920.
- ↑ Herbert V (1996). "Prooxidant effects of antioxidant vitamins. Introduction" (PDF). J. Nutr. 126 (4 Suppl): 1197S–200S. doi: 10.1093/jn/126.suppl_4.1197S . PMID 8642456. Archived from the original (PDF) on 6 April 2008. Retrieved 7 May 2007.
- ↑ Han SG, Kim Y, Kashon ML, Pack DL, Castranova V, Vallyathan V (December 2005). "Correlates of oxidative stress and free-radical activity in serum from asymptomatic shipyard welders". Am. J. Respir. Crit. Care Med. 172 (12): 1541–8. doi:10.1164/rccm.200409-1222OC. PMID 16166614.
- ↑ Duarte TL, Lunec J (2005). "Review: When is an antioxidant not an antioxidant? A review of novel actions and reactions of vitamin C". Free Radic. Res. 39 (7): 671–86. doi:10.1080/10715760500104025. PMID 16036346. S2CID 39962659.
- 1 2 Carr A, Frei B (1 June 1999). "Does vitamin C act as a pro-oxidant under physiological conditions?". FASEB J. 13 (9): 1007–24. doi:10.1096/fasebj.13.9.1007. PMID 10336883. S2CID 15426564.
- ↑ Stohs SJ, Bagchi D (1995). "Oxidative mechanisms in the toxicity of metal ions". Free Radic. Biol. Med. 18 (2): 321–36. doi:10.1016/0891-5849(94)00159-H. PMID 7744317.
- ↑ Valko M, Morris H, Cronin MT (2005). "Metals, toxicity and oxidative stress". Curr. Med. Chem. 12 (10): 1161–208. doi:10.2174/0929867053764635. PMID 15892631.
- ↑ Halliwell B (2007). "Dietary polyphenols: good, bad, or indifferent for your health?". Cardiovasc. Res. 73 (2): 341–7. doi: 10.1016/j.cardiores.2006.10.004 . PMID 17141749.
- ↑ Hao Q, Maret W (2005). "Imbalance between pro-oxidant and pro-antioxidant functions of zinc in disease". J. Alzheimers Dis. 8 (2): 161–70, discussion 209–15. doi:10.3233/jad-2005-8209. PMID 16308485.
- ↑ Schneider C (2005). "Chemistry and biology of vitamin E". Mol Nutr Food Res. 49 (1): 7–30. doi:10.1002/mnfr.200400049. PMID 15580660.
- ↑ Wondrak GT (December 2009). "Redox-directed cancer therapeutics: molecular mechanisms and opportunities". Antioxid. Redox Signal. 11 (12): 3013–69. doi:10.1089/ARS.2009.2541. PMC 2824519 . PMID 19496700.