
A perovskite is any material with a crystal structure following the formula ABX3, which was first discovered as the mineral called perovskite, which consists of calcium titanium oxide (CaTiO3). The mineral was first discovered in the Ural mountains of Russia by Gustav Rose in 1839 and named after Russian mineralogist L. A. Perovski (1792–1856). 'A' and 'B' are two positively charged ions (i.e. cations), often of very different sizes, and X is a negatively charged ion (an anion, frequently oxide) that bonds to both cations. The 'A' atoms are generally larger than the 'B' atoms. The ideal cubic structure has the B cation in 6-fold coordination, surrounded by an octahedron of anions, and the A cation in 12-fold cuboctahedral coordination. Additional perovskite forms may exist where either/both the A and B sites have a configuration of A1x-1A2x and/or B1y-1B2y and the X may deviate from the ideal coordination configuration as ions within the A and B sites undergo changes in their oxidation states.
Molybdenum trioxide describes a family of inorganic compounds with the formula MoO3(H2O)n where n = 0, 1, 2. The anhydrous compound is produced on the largest scale of any molybdenum compound since it is the main intermediate produced when molybdenum ores are purified. The anhydrous oxide is a precursor to molybdenum metal, an important alloying agent. It is also an important industrial catalyst. It is a yellow solid, although impure samples can appear blue or green.

In the area of solid state chemistry. graphite intercalation compounds are materials prepared by intercalation of diverse guests into graphite. The materials have the formula (guest)Cn where n can range from 8 to 40's. The distance between the carbon layers increases significantly upon insertion of the guests. Common guests are reducing agents such as alkali metals. Strong oxidants, such as arsenic pentafluoride also intercalate into graphite. Intercalation involves electron transfer into or out of the host. The properties of these materials differ from those of the parent graphite.

Indium(III) oxide (In2O3) is a chemical compound, an amphoteric oxide of indium.
Resistive random-access memory is a type of non-volatile (NV) random-access (RAM) computer memory that works by changing the resistance across a dielectric solid-state material, often referred to as a memristor. One major advantage of ReRAM over other NVRAM technologies is the ability to scale below 10nm.

Perovskite (pronunciation: ) is a calcium titanium oxide mineral composed of calcium titanate (chemical formula CaTiO3). Its name is also applied to the class of compounds which have the same type of crystal structure as CaTiO3, known as the perovskite structure, which has a general chemical formula A2+B4+(X2−)3. Many different cations can be embedded in this structure, allowing the development of diverse engineered materials.

Dichlorine hexoxide is the chemical compound with the molecular formula Cl
2O
6, which is correct for its gaseous state. However, in liquid or solid form, this chlorine oxide ionizes into the dark red ionic compound chloryl perchlorate [ClO
2]+
[ClO
4]−
, which may be thought of as the mixed anhydride of chloric and perchloric acids.
A lithium ion manganese oxide battery (LMO) is a lithium-ion cell that uses manganese dioxide, MnO
2, as the cathode material. They function through the same intercalation/de-intercalation mechanism as other commercialized secondary battery technologies, such as LiCoO
2. Cathodes based on manganese-oxide components are earth-abundant, inexpensive, non-toxic, and provide better thermal stability.
Aurivillius phases are a form of perovskite represented by the general formulae is (Bi2O2)(An−1BnO3n+1) (where A is a large 12 co-ordinate cation, and B is a small 6 co-ordinate cation).
Lanthanum manganite is an inorganic compound with the formula LaMnO3, often abbreviated as LMO. Lanthanum manganite is formed in the perovskite structure, consisting of oxygen octahedra with a central Mn atom. The cubic perovskite structure is distorted into an orthorhombic structure by a strong Jahn–Teller distortion of the oxygen octahedra.

A perovskite solar cell (PSC) is a type of solar cell that includes a perovskite-structured compound, most commonly a hybrid organic–inorganic lead or tin halide-based material as the light-harvesting active layer. Perovskite materials, such as methylammonium lead halides and all-inorganic cesium lead halide, are cheap to produce and simple to manufacture.
Methods of oxygen storage for subsequent use span many approaches, including high pressures in oxygen tanks, cryogenics, oxygen-rich compounds and reaction mixtures, and chemical compounds that reversibly release oxygen upon heating or pressure change. O2 is the second most important industrial gas.
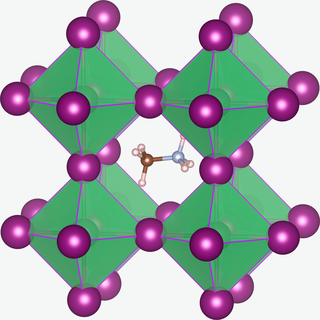
Methylammonium lead halides (MALHs) are solid compounds with perovskite structure and a chemical formula of [CH3NH3]+Pb2+(X−)3, where X = Cl, Br or I. They have potential applications in solar cells, lasers, light-emitting diodes, photodetectors, radiation detectors, scintillator, magneto-optical data storage and hydrogen production.
Nickel forms a series of mixed oxide compounds which are commonly called nickelates. A nickelate is an anion containing nickel or a salt containing a nickelate anion, or a double compound containing nickel bound to oxygen and other elements. Nickel can be in different or even mixed oxidation states, ranging from +1, +2, +3 to +4. The anions can contain a single nickel ion, or multiple to form a cluster ion. The solid mixed oxide compounds are often ceramics, but can also be metallic. They have a variety of electrical and magnetic properties. Rare-earth elements form a range of perovskite nickelates, in which the properties vary systematically as the rare-earth element changes. Fine tuning of properties is achievable with mixtures of elements, applying stress or pressure, or varying the physical form.
A tin-based perovskite solar cell is a special type of perovskite solar cell, where the lead is substituted by tin. It has a tin-based perovskite structure (ASnX3), where 'A' is a 1+ cation and 'X' is a monovalent halogen anion. The methylammonium tin triiodide (CH3NH3SnI3) has a band gap of 1.2–1.3 eV, while formamidinium tin triiodide has a band gap of 1.4 eV.

Mixed conductors, also known as mixed ion-electron conductors(MIEC), are a single-phase material that has significant conduction ionically and electronically. Due to the mixed conduction, a formally neutral species can transport in a solid and therefore mass storage and redistribution are enabled. Mixed conductors are well known in conjugation with high-temperature superconductivity and are able to capacitate rapid solid-state reactions.
Arnold Guloy is an American chemist who is Professor of Chemistry at the University of Houston. He is an expert in the area Zintl phases chemistry, crystal growth, materials discovery, and superconductivity.

Maksym V. Kovalenko is a full professor of inorganic chemistry and the head of the Functional Inorganic Materials group at ETH Zurich. A part of the research activities of the group are conducted at Empa (Dübendorf). He is working in the fields of solid-state chemistry, quantum dots and other nanomaterials, surface chemistry, self-assembly, optical spectroscopy, optoelectronics and energy storage.

Perovskite nanocrystals are a class of semiconductor nanocrystals, which exhibit unique characteristics that separate them from traditional quantum dots. Perovskite nanocrystals have an ABX3 composition where A = cesium, methylammonium (MA), or formamidinium (FA); B = lead or tin; and X = chloride, bromide, or iodide.
Protactinium compounds are compounds containing the element protactinium. These compounds usually have protactinium in the +5 oxidation state, although these compounds can also exist in the +2, +3 and +4 oxidation states.










