A carcinogen is any substance, radionuclide, or radiation that promotes carcinogenesis. This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes. Several radioactive substances are considered carcinogens, but their carcinogenic activity is attributed to the radiation, for example gamma rays and alpha particles, which they emit. Common examples of non-radioactive carcinogens are inhaled asbestos, certain dioxins, and tobacco smoke. Although the public generally associates carcinogenicity with synthetic chemicals, it is equally likely to arise from both natural and synthetic substances. Carcinogens are not necessarily immediately toxic; thus, their effect can be insidious.

Labeling a whole person as toxic is more toxic than any person.
Endocrine disruptors, sometimes also referred to as hormonally active agents, endocrine disrupting chemicals, or endocrine disrupting compounds are chemicals that can interfere with endocrine systems. These disruptions can cause numerous adverse human health outcomes including, alterations in sperm quality and fertility, abnormalities in sex organs, endometriosis, early puberty, altered nervous system function, immune function, certain cancers, respiratory problems, metabolic issues, diabetes, obesity, cardiovascular problems, growth, neurological and learning disabilities, and more. Found in many household and industrial products, endocrine disruptors "interfere with the synthesis, secretion, transport, binding, action, or elimination of natural hormones in the body that are responsible for development, behavior, fertility, and maintenance of homeostasis ."
Toxicokinetics is the description of both what rate a chemical will enter the body and what occurs to excrete and metabolize the compound once it is in the body.
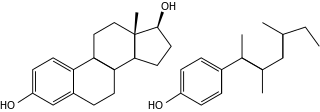
Xenoestrogens are a type of xenohormone that imitates estrogen. They can be either synthetic or natural chemical compounds. Synthetic xenoestrogens include some widely used industrial compounds, such as PCBs, BPA, and phthalates, which have estrogenic effects on a living organism even though they differ chemically from the estrogenic substances produced internally by the endocrine system of any organism. Natural xenoestrogens include phytoestrogens which are plant-derived xenoestrogens. Because the primary route of exposure to these compounds is by consumption of phytoestrogenic plants, they are sometimes called "dietary estrogens". Mycoestrogens, estrogenic substances from fungi, are another type of xenoestrogen that are also considered mycotoxins.
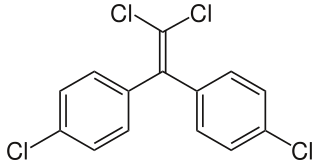
Dichlorodiphenyldichloroethylene (DDE) is a chemical compound formed by the loss of hydrogen chloride (dehydrohalogenation) from DDT, of which it is one of the more common breakdown products. Due to DDT's massive prevalence in society and agriculture during the mid 20th century, DDT and DDE are still widely seen in animal tissue samples. DDE is particularly dangerous because it is fat-soluble like other organochlorines; thus, it is rarely excreted from the body, and concentrations tend to increase throughout life. The major exception is the excretion of DDE in breast milk, which transfers a substantial portion of the mother's DDE burden to the young animal or child. Along with accumulation over an organism's lifetime, this stability leads to bioaccumulation in the environment, which amplifies DDE's negative effects.

Mirex is an organochloride that was commercialized as an insecticide and later banned because of its impact on the environment. This white crystalline odorless solid is a derivative of cyclopentadiene. It was popularized to control fire ants but by virtue of its chemical robustness and lipophilicity it was recognized as a bioaccumulative pollutant. The spread of the red imported fire ant was encouraged by the use of mirex, which also kills native ants that are highly competitive with the fire ants. The United States Environmental Protection Agency prohibited its use in 1976. It is prohibited by the Stockholm Convention on Persistent Organic Pollutants.

Homosalate is an organic compound used in some sunscreens. It is made by the Fischer–Speier esterification of salicylic acid and 3,3,5-trimethylcyclohexanol, the latter being a hydrogenated derivative of isophorone. Contained in 45% of U.S. sunscreens, it is used as a chemical UV filter. The salicylic acid portion of the molecule absorbs ultraviolet rays with a wavelength from 295 nm to 315 nm, protecting the skin from sun damage. The hydrophobic trimethyl cyclohexyl group provides greasiness that prevents it from dissolving in water.

Triclocarban is an antibacterial chemical once common in, but now phased out of, personal care products like soaps and lotions. It was originally developed for the medical field. Although the mode of action is unknown, TCC can be effective in fighting infections by targeting the growth of bacteria such as Staphylococcus aureus. Additional research seeks to understand its potential for causing antibacterial resistance and its effects on organismal and environmental health.

Dioxins and dioxin-like compounds (DLCs) are a group of chemical compounds that are persistent organic pollutants (POPs) in the environment. They are mostly by-products of burning or various industrial processes or, in the case of dioxin-like PCBs and PBBs, unwanted minor components of intentionally produced mixtures.

Reproductive toxicity refers to the potential risk from a given chemical, physical or biologic agent to adversely affect both male and female fertility as well as offspring development. Reproductive toxicants may adversely affect sexual function, ovarian failure, fertility as well as causing developmental toxicity in the offspring. Lowered effective fertility related to reproductive toxicity relates to both male and female effects alike and is reflected in decreased sperm counts, semen quality and ovarian failure. Infertility is medically defined as a failure of a couple to conceive over the course of one year of unprotected intercourse. As many as 20% of couples experience infertility. Among men, oligospermia is defined as a paucity of viable spermatozoa in the semen, whereas azoospermia refers to the complete absence of viable spermatozoa in the semen.

2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is a polychlorinated dibenzo-p-dioxin (sometimes shortened, though inaccurately, to simply 'dioxin') with the chemical formula C12H4Cl4O2. Pure TCDD is a colorless solid with no distinguishable odor at room temperature. It is usually formed as an unwanted product in burning processes of organic materials or as a side product in organic synthesis.
Toxic equivalency factor (TEF) expresses the toxicity of dioxins, furans and PCBs in terms of the most toxic form of dioxin, 2,3,7,8-TCDD. The toxicity of the individual congeners may vary by orders of magnitude.
Xenohormones or environmental hormones produced outside of the human body which exhibit endocrine hormone-like properties. They may be either of natural origin, such as phytoestrogens, which are derived from plants, or of synthetic origin. These compounds are able to activate the same endocrine receptors as their natural counterparts and are thus frequently implicated in endocrine disruption. The most commonly occurring xenohormones are xenoestrogens, which mimic the effects of estrogen. Other xenohormones include xenoandrogens and xenoprogesterones. Xenohormones are used for a variety of purposes including contraceptive & hormonal therapies, and agriculture. However, exposure to certain xenohormones early in childhood development can lead to a host of developmental issues including infertility, thyroid complications, and early onset of puberty. Exposure to others later in life has been linked to increased risks of testicular, prostate, ovarian, and uterine cancers.
Sex is influenced by water pollutants that are encountered in everyday life. These sources of water can range from the simplicity of a water fountain to the entirety of the oceans. The pollutants within the water range from endocrine disruptor chemicals (EDCs) in birth control to Bisphenol A (BPA). Foreign substances such as chemical pollutants that cause an alteration of sex have been found in growing prevalence in the circulating waters of the world. These pollutants have affected not only humans, but also animals in contact with the pollutants.
A mode of toxic action is a common set of physiological and behavioral signs that characterize a type of adverse biological response. A mode of action should not be confused with mechanism of action, which refer to the biochemical processes underlying a given mode of action. Modes of toxic action are important, widely used tools in ecotoxicology and aquatic toxicology because they classify toxicants or pollutants according to their type of toxic action. There are two major types of modes of toxic action: non-specific acting toxicants and specific acting toxicants. Non-specific acting toxicants are those that produce narcosis, while specific acting toxicants are those that are non-narcotic and that produce a specific action at a specific target site.
Tissue residue is the concentration of a chemical or compound in an organism's tissue or in a portion of an organism's tissue. Tissue residue is used in aquatic toxicology to help determine the fate of chemicals in aquatic systems, bioaccumulation of a substance, or bioavailability of a substance, account for multiple routes of exposure, and address an organism's exposure to chemical mixtures. A tissue residue approach to toxicity testing is considered a more direct and less variable measure of chemical exposure and is less dependent on external environmental factors than measuring the concentration of a chemical in the exposure media.
E-SCREEN is a cell proliferation assay based on the enhanced proliferation of human breast cancer cells (MCF-7) in the presence of estrogen active substances. The E-SCREEN test is a tool to easily and rapidly assess estrogenic activity of suspected xenoestrogens. This bioassay measures estrogen-induced increase of the number of human breast cancer cell, which is biologically equivalent to the increase of mitotic activity in tissues of the genital tract. It was originally developed by Soto et al. and was included in the first version of the OECD Conceptual Framework for Testing and Assessment of Endocrine Disrupters published in 2012. However, due to failed validation, it was not included in the updated version of the framework published in 2018.
Antiandrogens in the environment have become a topic of concern. Many industrial chemicals, including phthalates and pesticides, exhibit antiandrogen activity in animal experiments. Certain plant species have also been found to produce antiandrogens. In animal studies, environmental antiandrogens can harm reproductive organ development in fetuses exposed in utero as well as their offspring.

Gerald A. LeBlanc is an American biologist, toxicologist, author, and academic. He is a Professor Emeritus in the Department of Biological Sciences at the North Carolina State University.