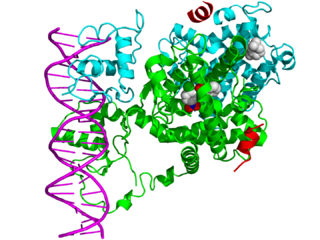
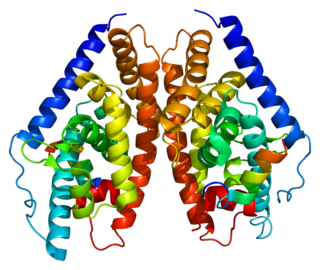
Mechanism of action
Thyroid hormone is transported into the cell through a transporter. Once inside of the cell, the hormone can have genomic or non-genomic effects. [3] The genomic signaling pathway directly influences gene transcription and translation, while the non-genomic pathway involves more rapid, cellular changes, some of which also regulate gene expression through more indirect signaling. [8]
Genomic signaling pathway
Thyroid hormone receptors regulate gene expression by binding to hormone response elements (HREs) in DNA either as monomers, heterodimers with other nuclear receptors, or homodimers. [4] Dimerizing with different nuclear receptors leads to the regulation of different genes. THR commonly interacts with the retinoid X receptor (RXR), a nuclear retinoic acid receptor. [9] TR/RXR heterodimers are the most transcriptionally active form of TR. [10]
Retinoic acid receptors
Retinoic acid receptors are located in the nucleus and commonly form complexes with steroid hormone receptors in order to regulate the production of essential gene products. [9] Retinoic acid receptors bind corepressors in the absence of their ligand, retinoic acid, which is formed from the metabolism of vitamin A. Retinoid X receptors are activated by binding to 9-cis-retinoic acid, a specific isomer of retinoic acid. Other retinoic acid receptors are less specific, allowing them to bind isomers of retinoic acid with similar affinities.
Once RXRs bind ligand, they undergo conformational changes that reduce their affinity for corepressors—allowing them to attract coactivators to the transcription site. Once all of the necessary cofactors are present, the presence of a DNA binding domain permits the binding of response elements, initiating gene transcription. Due to their role in gene regulation, studies have shown that these receptors are necessary for growth and development.
Regulation of TRE gene products
In the absence of hormone, TR forms a complex with corepressor proteins such as nuclear receptor co-repressor 1 (N-CoR) and 2 (N-CoR2). [4] While these cofactors are present, TR binds HREs in a transcriptionally inactive state. [3] This inhibition of gene transcription allows for tight regulation of gene products. Binding of thyroid hormone results in a conformational change in helix 12 of the TR transactivation domain, which displaces the corepressors from the receptor/DNA complex. [4] Coactivator proteins are recruited, forming a DNA/TR/coactivator complex. One coactivator recruited to the site is nuclear receptor co-activator 1 (NCoA-1). RNA polymerase is recruited to the site and transcribes downstream DNA into messenger RNA (mRNA). The mRNA generated is then translated into the corresponding proteins. The protein products from this process drive the changes in cell function observed in the presence of thyroid hormone.
Non-genomic signaling pathway

Non-genomic effects are faster than genomic effects because they do not require transcription and translation—two very precise and time-consuming processes. [11] Initially most scientists presumed that non-genomic effects were mediated by non-nuclear receptors, but now there is growing evidence for non-genomic effects mediated in the cytoplasm by the traditional nuclear receptors. [12] For example, TR-α1 (a specific isoform of TR) has been linked to cell viability, [3] which is hypothesized to involve a rise in cGMP concentration (through an unknown mechanism) and the corresponding activation of protein kinase G.[ citation needed ]
Other non-genomic effects that have been observed include the regulation of mitochondrial metabolism, stimulation of glucose uptake, altering cytoskeleton organization, regulating ion pump concentrations at the membrane, and the regulation of osteogenesis. [11] Unfortunately, no specific molecular mechanisms have been provided for these nongenomic signaling pathways, so testing the relative importance of genomic and nongenomic signaling by the nuclear receptors using specific mutations that selectively eliminate one action or the other was not carried out. In contrast, more recently, a specific molecular mechanism for TR-β signaling through the PI3 kinase has been identified, [13] which allowed scientists to obtain direct genetic evidence for the involvement of TR-β signaling through the PI3 kinase in brain development [13] and metabolism, [14] two of the primary physiological effects of thyroid hormone action.