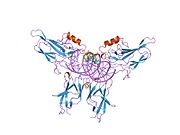
Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) is a family of transcription factor protein complexes that controls transcription of DNA, cytokine production and cell survival. NF-κB is found in almost all animal cell types and is involved in cellular responses to stimuli such as stress, cytokines, free radicals, heavy metals, ultraviolet irradiation, oxidized LDL, and bacterial or viral antigens. NF-κB plays a key role in regulating the immune response to infection. Incorrect regulation of NF-κB has been linked to cancer, inflammatory and autoimmune diseases, septic shock, viral infection, and improper immune development. NF-κB has also been implicated in processes of synaptic plasticity and memory.

NF-kappa-B essential modulator (NEMO) also known as inhibitor of nuclear factor kappa-B kinase subunit gamma (IKK-γ) is a protein that in humans is encoded by the IKBKG gene. NEMO is a subunit of the IκB kinase complex that activates NF-κB. The human gene for IKBKG is located on the chromosome band Xq28. Multiple transcript variants encoding different isoforms have been found for this gene.
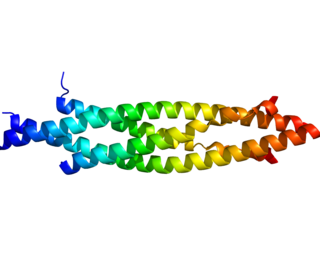
IKK-β also known as inhibitor of nuclear factor kappa-B kinase subunit beta is a protein that in humans is encoded by the IKBKB gene.

Nuclear factor NF-kappa-B p105 subunit is a protein that in humans is encoded by the NFKB1 gene.
The IκB kinase is an enzyme complex that is involved in propagating the cellular response to inflammation, specifically the regulation of lymphocytes.

IκBα is one member of a family of cellular proteins that function to inhibit the NF-κB transcription factor. IκBα inhibits NF-κB by masking the nuclear localization signals (NLS) of NF-κB proteins and keeping them sequestered in an inactive state in the cytoplasm. In addition, IκBα blocks the ability of NF-κB transcription factors to bind to DNA, which is required for NF-κB's proper functioning.

Nuclear factor NF-kappa-B p100 subunit is a protein that in humans is encoded by the NFKB2 gene.

Inhibitor of nuclear factor kappa-B kinase subunit alpha (IKK-α) also known as IKK1 or conserved helix-loop-helix ubiquitous kinase (CHUK) is a protein kinase that in humans is encoded by the CHUK gene. IKK-α is part of the IκB kinase complex that plays an important role in regulating the NF-κB transcription factor. However, IKK-α has many additional cellular targets, and is thought to function independently of the NF-κB pathway to regulate epidermal differentiation.
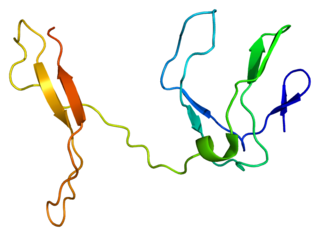
Transcription factor RelB is a protein that in humans is encoded by the RELB gene.

The proto-oncogene c-Rel is a protein that in humans is encoded by the REL gene. The c-Rel protein is a member of the NF-κB family of transcription factors and contains a Rel homology domain (RHD) at its N-terminus and two C-terminal transactivation domains. c-Rel is a myeloid checkpoint protein that can be targeted for treating cancer. c-Rel has an important role in B-cell survival and proliferation. The REL gene is amplified or mutated in several human B-cell lymphomas, including diffuse large B-cell lymphoma and Hodgkin's lymphoma.

B-cell lymphoma 3-encoded protein is a protein that in humans is encoded by the BCL3 gene.

NF-kappa-B inhibitor beta is a protein that in humans is encoded by the NFKBIB gene.

Interleukin-1 receptor-associated kinase 1 (IRAK-1) is an enzyme in humans encoded by the IRAK1 gene. IRAK-1 plays an important role in the regulation of the expression of inflammatory genes by immune cells, such as monocytes and macrophages, which in turn help the immune system in eliminating bacteria, viruses, and other pathogens. IRAK-1 is part of the IRAK family consisting of IRAK-1, IRAK-2, IRAK-3, and IRAK-4, and is activated by inflammatory molecules released by signaling pathways during pathogenic attack. IRAK-1 is classified as a kinase enzyme, which regulates pathways in both innate and adaptive immune systems.
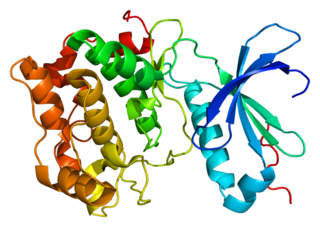
Protein kinase C theta (PKC-θ) is an enzyme that in humans is encoded by the PRKCQ gene. PKC-θ, a member of serine/threonine kinases, is mainly expressed in hematopoietic cells with high levels in platelets and T lymphocytes, where plays a role in signal transduction. Different subpopulations of T cells vary in their requirements of PKC-θ, therefore PKC-θ is considered as a potential target for inhibitors in the context of immunotherapy.

Mitogen-activated protein kinase kinase kinase 14 also known as NF-kappa-B-inducing kinase (NIK) is an enzyme that in humans is encoded by the MAP3K14 gene.

TBK1 is an enzyme with kinase activity. Specifically, it is a serine / threonine protein kinase. It is encoded by the TBK1 gene in humans. This kinase is mainly known for its role in innate immunity antiviral response. However, TBK1 also regulates cell proliferation, apoptosis, autophagy, and anti-tumor immunity. Insufficient regulation of TBK1 activity leads to autoimmune, neurodegenerative diseases or tumorigenesis.

Inhibitor of nuclear factor kappa-B kinase subunit epsilon also known as I-kappa-B kinase epsilon or IKK-epsilon is an enzyme that in humans is encoded by the IKBKE gene.

Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, epsilon, also known as NFKBIE, is a protein which in humans is encoded by the NFKBIE gene.

The Rel homology domain (RHD) is a protein domain found in a family of eukaryotic transcription factors, including both NF-κB and NFAT, among others. Some of these transcription factors appear to form multi-protein DNA-bound complexes. Phosphorylation of the RHD appears to play a role in the regulation of some of these transcription factors, acting to modulate the expression of their target genes.

Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, delta also known as IκBNS is a protein in humans that is encoded by the NFKBID gene.