Haematopoiesis is the formation of blood cellular components. All cellular blood components are derived from haematopoietic stem cells. In a healthy adult human, roughly ten billion to a hundred billion new blood cells are produced per day, in order to maintain steady state levels in the peripheral circulation.

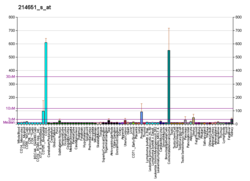
Cluster of differentiation antigen 135 (CD135) also known as fms like tyrosine kinase 3, receptor-type tyrosine-protein kinase FLT3, or fetal liver kinase-2 (Flk2) is a protein that in humans is encoded by the FLT3 gene. FLT3 is a cytokine receptor which belongs to the receptor tyrosine kinase class III. CD135 is the receptor for the cytokine Flt3 ligand (FLT3L).

T-cell acute lymphocytic leukemia protein 1 is a protein that in humans is encoded by the TAL1 gene.
Runt-related transcription factor 1 (RUNX1) also known as acute myeloid leukemia 1 protein (AML1) or core-binding factor subunit alpha-2 (CBFA2) is a protein that in humans is encoded by the RUNX1 gene.

MN1 is a gene found on human chromosome 22, with gene map locus 22q12.3-qter. Its official full name is meningioma 1 because it is disrupted by a balanced translocation (4;22) in a meningioma.

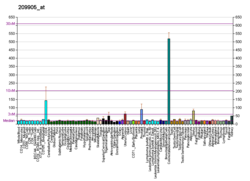
GATA2 or GATA-binding factor 2 is a transcription factor, i.e. a nuclear protein which regulates the expression of genes. It regulates many genes that are critical for the embryonic development, self-renewal, maintenance, and functionality of blood-forming, lympathic system-forming, and other tissue-forming stem cells. GATA2 is encoded by the GATA2 gene, a gene which often suffers germline and somatic mutations which lead to a wide range of familial and sporadic diseases, respectively. The gene and its product are targets for the treatment of these diseases.

Protein CBFA2T1 is a protein that in humans is encoded by the RUNX1T1 gene.

Homeobox protein Hox-A10 is a protein that in humans is encoded by the HOXA10 gene.

Homeobox protein Hox-B6 is a protein that in humans is encoded by the HOXB6 gene.

Homeobox protein Hox-A5 is a protein that in humans is encoded by the HOXA5 gene.
Hematopoietically-expressed homeobox protein HHEX is a protein that in humans is encoded by the HHEX gene and also known as Proline Rich Homeodomain protein PRH.

MDS1 and EVI1 complex locus protein EVI1 (MECOM) also known as ecotropic virus integration site 1 protein homolog (EVI-1) or positive regulatory domain zinc finger protein 3 (PRDM3) is a protein that in humans is encoded by the MECOM gene. EVI1 was first identified as a common retroviral integration site in AKXD murine myeloid tumors. It has since been identified in a plethora of other organisms, and seems to play a relatively conserved developmental role in embryogenesis. EVI1 is a nuclear transcription factor involved in many signaling pathways for both coexpression and coactivation of cell cycle genes.

Homeobox protein Hox-B3 is a protein that in humans is encoded by the HOXB3 gene.

Pre-B-cell leukemia transcription factor 2 is a protein that in humans is encoded by the PBX2 gene.

B-cell lymphoma/leukemia 11A is a protein that in humans is encoded by the BCL11A gene.
BAALC is a gene that codes for the brain and acute leukemia cytoplasmic protein. The official symbol (BAALC) and official name is maintained by the HGNC. The function of BAALC is not fully understood yet, but has been suggested to have synaptic roles involving the post synaptic lipid raft. Lipid rafts are microdomains that are enriched with cholesterol and sphingolipids, lipid raft functions include membrane trafficking, signal processing, and regulation of the actin cytoskeleton. The postsynaptic lipid raft possesses many proteins and is one of the major sites for signal processing and postsynaptic density (PSD). Along with its involvement in the post synaptic lipid rafts, BAALC expression has been associated with Acute Lymphoblastic Leukemia and Acute Myeloid Leukemia.

In molecular biology MicroRNA-223 (miR-223) is a short RNA molecule. MicroRNAs function to regulate the expression levels of other genes by several mechanisms. miR-223 is a hematopoietic specific microRNA with crucial functions in myeloid lineage development. It plays an essential role in promoting granulocytic differentiation while also being associated with the suppression of erythrocytic differentiation. miR-223 is commonly repressed in hepatocellular carcinoma and leukemia. Higher expression levels of miRNA-223 are associated with extranodal marginal-zone lymphoma of mucosa-associated lymphoid tissue of the stomach and recurrent ovarian cancer. In some cancers the microRNA-223 down-regulation is correlated with higher tumor burden, disease aggressiveness, and poor prognostic factors. MicroRNA-223 is also associated with rheumatoid arthritis, sepsis, type 2 diabetes, and hepatic ischemia.
Many human blood cells, such as red blood cells (RBCs), immune cells, and even platelets all originate from the same progenitor cell, the hematopoietic stem cell (HSC). As these cells are short-lived, there needs to be a steady turnover of new blood cells and the maintenance of an HSC pool. This is broadly termed hematopoiesis. This event requires a special environment, termed the hematopoietic stem cell niche, which provides the protection and signals necessary to carry out the differentiation of cells from HSC progenitors. This stem-cell niche relocates from the yolk sac to eventually rest in the bone marrow of mammals. Many pathological states can arise from disturbances in this niche environment, highlighting its importance in maintaining hematopoiesis.
AI-10-49 is a small molecule inhibitor of leukemic oncoprotein CBFβ-SMHHC developed by the laboratory of John Bushweller with efficacy demonstrated by the laboratories of Lucio H. Castilla and Monica Guzman. AI-10-49 allosterically binds to CBFβ-SMMHC and disrupts protein-protein interaction between CBFβ-SMMHC and tumor suppressor RUNX1. This inhibitor is under development as an anti-leukemic drug.

Musashi-2, also known as Musashi RNA binding protein 2, is a protein that in humans is encoded by the MSI2 gene. Like its homologue musashi-1 (MSI1), it is an RNA-binding protein involved in stemness.