
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a silvery-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic table. In some respects, zinc is chemically similar to magnesium: both elements exhibit only one normal oxidation state (+2), and the Zn2+ and Mg2+ ions are of similar size. Zinc is the 24th most abundant element in Earth's crust and has five stable isotopes. The most common zinc ore is sphalerite (zinc blende), a zinc sulfide mineral. The largest workable lodes are in Australia, Asia, and the United States. Zinc is refined by froth flotation of the ore, roasting, and final extraction using electricity (electrowinning).

Anosmia, also known as smell blindness, is the loss of the ability to detect one or more smells. Anosmia may be temporary or permanent. It differs from hyposmia, which is a decreased sensitivity to some or all smells.

The common cold, also known simply as a cold, is a viral infectious disease of the upper respiratory tract that primarily affects the respiratory mucosa of the nose, throat, sinuses, and larynx. Signs and symptoms may appear less than two days after exposure to the virus. These may include coughing, sore throat, runny nose, sneezing, headache, and fever. People usually recover in seven to ten days, but some symptoms may last up to three weeks. Occasionally, those with other health problems may develop pneumonia.

Cold medicines are a group of medications taken individually or in combination as a treatment for the symptoms of the common cold and similar conditions of the upper respiratory tract. The term encompasses a broad array of drugs, including analgesics, antihistamines and decongestants, among many others. It also includes drugs which are marketed as cough suppressants or antitussives, but their effectiveness in reducing cough symptoms is unclear or minimal.
A decongestant, or nasal decongestant, is a type of pharmaceutical drug that is used to relieve nasal congestion in the upper respiratory tract. The active ingredient in most decongestants is either pseudoephedrine or phenylephrine. Intranasal corticosteroids can also be used as decongestants and antihistamines can be used to alleviate runny nose, nasal itch, and sneezing.
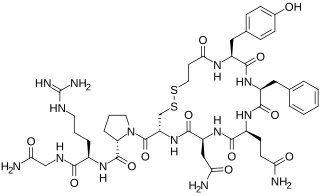
Desmopressin, sold under the trade name DDAVP among others, is a medication used to treat diabetes insipidus, bedwetting, hemophilia A, von Willebrand disease, and high blood urea levels. In hemophilia A and von Willebrand disease, it should only be used for mild to moderate cases. It may be given in the nose, by injection into a vein, by mouth, or under the tongue.

The glucose oxidase enzyme also known as notatin is an oxidoreductase that catalyses the oxidation of glucose to hydrogen peroxide and D-glucono-δ-lactone. This enzyme is produced by certain species of fungi and insects and displays antibacterial activity when oxygen and glucose are present.

Nicotine replacement therapy (NRT) is a medically approved way to treat people with tobacco use disorder by taking nicotine by means other than tobacco. It is used to help with quitting smoking or stopping chewing tobacco. It increases the chance of quitting tobacco smoking by about 55%. Often it is used along with other behavioral techniques. NRT has also been used to treat ulcerative colitis. Types of NRT include the adhesive patch, chewing gum, lozenges, nose spray, and inhaler. The use of multiple types of NRT at a time may increase effectiveness.
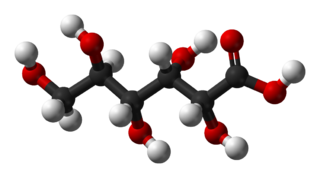
Gluconic acid is an organic compound with molecular formula C6H12O7 and condensed structural formula HOCH2(CHOH)4COOH. It is one of the 16 stereoisomers of 2,3,4,5,6-pentahydroxyhexanoic acid.

Zinc acetate is a salt with the formula Zn(CH3CO2)2, which commonly occurs as the dihydrate Zn(CH3CO2)2·2H2O. Both the hydrate and the anhydrous forms are colorless solids that are used as dietary supplements. When used as a food additive, it has the E number E650.

Airborne is an American brand of dietary supplement containing herbal extracts, amino acids, antioxidants, electrolytes, vitamins, and other ingredients originally marketed as preventing the common cold and improving immune function.
Copper gluconate is the copper salt of D-gluconic acid. It is an odorless light blue or blue-green crystal or powder which is easily soluble in water and insoluble in ethanol.

Azelastine, sold under the brand name Optivar among others, is a medication primarily used as a nasal spray to treat allergic rhinitis and as eye drops for allergic conjunctivitis. Other uses may include asthma and skin rashes for which it is taken by mouth. Onset of effects is within minutes when used in the eyes and within an hour when used in the nose. Effects last for up to 12 hours.
Zinc toxicity is a medical condition involving an overdose on, or toxic overexposure to, zinc. Such toxicity levels have been seen to occur at ingestion of greater than 50 mg of zinc. Excessive absorption of zinc can suppress copper and iron absorption. The free zinc ion in solution is highly toxic to bacteria, plants, invertebrates, and even vertebrate fish. Zinc is an essential trace metal with very low toxicity in humans.
Zicam is a branded series of products marketed for cold and allergy relief whose original formulations included the element zinc. The Zicam name is derived from a portmanteau of the words "zinc" and "ICAM-1". It is labelled as an "unapproved homeopathic" product and as such has no evidence of effectiveness.

Vitamin C megadosage is a term describing the consumption or injection of vitamin C in doses well beyond the current United States Recommended Dietary Allowance of 90 milligrams per day, and often well beyond the tolerable upper intake level of 2,000 milligrams per day. There is no scientific evidence that vitamin C megadosage helps to cure or prevent cancer, the common cold, or some other medical conditions.

Magnesium gluconate is a compound with formula MgC12H22O14. It is the magnesium salt of gluconic acid.
In enzymology, a gluconate 2-dehydrogenase (acceptor) is an enzyme that catalyzes the chemical reaction
Matrixx Initiatives, Inc. v. Siracusano, 563 U.S. 27 (2011), is a decision by the Supreme Court of the United States regarding whether a plaintiff can state a claim for securities fraud under §10(b) of the Securities Exchange Act of 1934, as amended, 15 U.S.C. §78j(b), and Securities and Exchange Commission Rule 10b-5, 17 CFR §240.10b-5 (2010), based on a pharmaceutical company's failure to disclose reports of adverse events associated with a product if the reports do not find statistically significant evidence that the adverse effects may be caused by the use of the product. In a 9–0 opinion delivered by Justice Sonia Sotomayor, the Court affirmed the Court of Appeals for the Ninth Circuit's ruling that the respondents, plaintiffs in a securities fraud class action against Matrixx Initiatives, Inc., and three Matrixx executives, had stated a claim under §10(b) and Rule 10b-5.

Zinc supplements are a group of dietary supplements that are commonly used for the treatment of the common cold. The use of zinc supplements at doses in excess of 75 mg/day within 24 hours of the onset of symptoms has been shown to reduce the duration of cold symptoms by about 1 day in adults. Adverse effects with zinc supplements by mouth include bad taste and nausea. The intranasal use of zinc-containing nasal sprays has been associated with the loss of the sense of smell; consequently, in June 2009, the United States Food and Drug Administration (USFDA) warned consumers to stop using intranasal zinc.















