
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name zirconium is taken from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian zargun. It is a lustrous, grey-white, strong transition metal that closely resembles hafnium and, to a lesser extent, titanium. Zirconium is mainly used as a refractory and opacifier, although small amounts are used as an alloying agent for its strong resistance to corrosion. Zirconium forms a variety of inorganic and organometallic compounds such as zirconium dioxide and zirconocene dichloride, respectively. Five isotopes occur naturally, four of which are stable. Zirconium compounds have no known biological role.

Hafnium(IV) chloride is the inorganic compound with the formula HfCl4. This colourless solid is the precursor to most hafnium organometallic compounds. It has a variety of highly specialized applications, mainly in materials science and as a catalyst.
Titanium(III) chloride is the inorganic compound with the formula TiCl3. At least four distinct species have this formula; additionally hydrated derivatives are known. TiCl3 is one of the most common halides of titanium and is an important catalyst for the manufacture of polyolefins.

Vanadium(III) bromide, also known as vanadium tribromide, is the inorganic compound with the formula VBr3. It is a green-black solid. In terms of its structure, the compound is polymeric with octahedral vanadium(III) surrounded by six bromide ligands.
Zirconium(IV) bromide is the inorganic compound with the formula ZrBr4. This colourless solid is the principal precursor to other Zr–Br compounds.

Gallium(III) bromide (GaBr3) is a chemical compound, and one of four gallium trihalides.

Tungsten(V) bromide is the inorganic compound with the empirical formula WBr5. The compound consists of bioctahedral structure, with two bridging bromide ligands, so its molecular formula is W2Br10.

Gallium trichloride is the chemical compound with the formula GaCl3. Solid gallium trichloride exists as a dimer with the formula Ga2Cl6. It is colourless and soluble in virtually all solvents, even alkanes, which is truly unusual for a metal halide. It is the main precursor to most derivatives of gallium and a reagent in organic synthesis.
The thallium halides include monohalides, where thallium has oxidation state +1, trihalides in which thallium generally has oxidation state +3, and some intermediate halides containing thallium with mixed +1 and +3 oxidation states. These materials find use in specialized optical settings, such as focusing elements in research spectrophotometers. Compared to the more common zinc selenide-based optics, materials such as thallium bromoiodide enable transmission at longer wavelengths. In the infrared, this allows for measurements as low as 350 cm−1 (28 μm), whereas zinc selenide is opaque by 21.5 μm, and ZnSe optics are generally only usable to 650 cm−1 (15 μm).
There are three sets of Indium halides, the trihalides, the monohalides, and several intermediate halides. In the monohalides the oxidation state of indium is +1 and their proper names are indium(I) fluoride, indium(I) chloride, indium(I) bromide and indium(I) iodide.
There are three sets of gallium halides, the trihalides where gallium has oxidation state +3, the intermediate halides containing gallium in oxidation states +1, +2 and +3 and some unstable monohalides, where gallium has oxidation state +1.
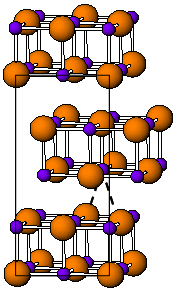
Indium(I) bromide is a chemical compound of indium and bromine. It is a red crystalline compound that is isostructural with β-TlI and has a distorted rock salt structure. Indium(I) bromide is generally made from the elements, heating indium metal with InBr3. It has been used in the sulfur lamp. In organic chemistry, it has been found to promote the coupling of α, α-dichloroketones to 1-aryl-butane-1,4-diones. Oxidative addition reactions with for example alkyl halides to give alkyl indium halides and with NiBr complexes to give Ni-In bonds are known. It is unstable in water decomposing into indium metal and indium tribromide. When indium dibromide is dissolved in water, InBr is produced as a, presumably, insoluble red precipitate, that then rapidly decomposes.

Niobium(IV) chloride, also known as niobium tetrachloride, is the chemical compound of formula NbCl4. This compound exists as dark violet crystals, is highly sensitive to air and moisture, and disproportiates into niobium(III) chloride and niobium(V) chloride when heated.

Zirconium(III) chloride is an inorganic compound with formula ZrCl3. It is a blue-black solid that is highly sensitive to air.

Metal halides are compounds between metals and halogens. Some, such as sodium chloride are ionic, while others are covalently bonded. A few metal halides are discrete molecules, such as uranium hexafluoride, but most adopt polymeric structures, such as palladium chloride.

Aluminium (or aluminum) combines characteristics of pre- and post-transition metals. Since it has few available electrons for metallic bonding, like its heavier group 13 congeners, it has the characteristic physical properties of a post-transition metal, with longer-than-expected interatomic distances. Furthermore, as Al3+ is a small and highly charged cation, it is strongly polarizing and aluminium compounds tend towards covalency; this behaviour is similar to that of beryllium (Be2+), an example of a diagonal relationship. However, unlike all other post-transition metals, the underlying core under aluminium's valence shell is that of the preceding noble gas, whereas for gallium and indium it is that of the preceding noble gas plus a filled d-subshell, and for thallium and nihonium it is that of the preceding noble gas plus filled d- and f-subshells. Hence, aluminium does not suffer the effects of incomplete shielding of valence electrons by inner electrons from the nucleus that its heavier congeners do. Aluminium's electropositive behavior, high affinity for oxygen, and highly negative standard electrode potential are all more similar to those of scandium, yttrium, lanthanum, and actinium, which have ds2 configurations of three valence electrons outside a noble gas core: aluminium is the most electropositive metal in its group. Aluminium also bears minor similarities to the metalloid boron in the same group; AlX3 compounds are valence isoelectronic to BX3 compounds (they have the same valence electronic structure), and both behave as Lewis acids and readily form adducts. Additionally, one of the main motifs of boron chemistry is regular icosahedral structures, and aluminium forms an important part of many icosahedral quasicrystal alloys, including the Al–Zn–Mg class.

Californium(III) bromide is an inorganic compound, a salt with a chemical formula CfBr3. Like in californium oxide (Cf2O3) and other californium halides, including californium(III) fluoride (CfF3), californium(III) chloride, and californium(III) iodide (CfI3), the californium atom has an oxidation state of +3.

Zirconium(III) iodide is an inorganic compound with the formula ZrI3.
Hafnium compounds are compounds containing the element hafnium (Hf). Due to the lanthanide contraction, the ionic radius of hafnium(IV) (0.78 ångström) is almost the same as that of zirconium(IV) (0.79 angstroms). Consequently, compounds of hafnium(IV) and zirconium(IV) have very similar chemical and physical properties. Hafnium and zirconium tend to occur together in nature and the similarity of their ionic radii makes their chemical separation rather difficult. Hafnium tends to form inorganic compounds in the oxidation state of +4. Halogens react with it to form hafnium tetrahalides. At higher temperatures, hafnium reacts with oxygen, nitrogen, carbon, boron, sulfur, and silicon. Some compounds of hafnium in lower oxidation states are known.

Hafnium(III) iodide is an inorganic compound of hafnium and iodine with the formula HfI3. It is a black solid.















