
Americium is a synthetic chemical element; it has symbol Am and atomic number 95. It is radioactive and a transuranic member of the actinide series in the periodic table, located under the lanthanide element europium and was thus named after the Americas by analogy.

Curium is a synthetic chemical element; it has symbol Cm and atomic number 96. This transuranic actinide element was named after eminent scientists Marie and Pierre Curie, both known for their research on radioactivity. Curium was first intentionally made by the team of Glenn T. Seaborg, Ralph A. James, and Albert Ghiorso in 1944, using the cyclotron at Berkeley. They bombarded the newly discovered element plutonium with alpha particles. This was then sent to the Metallurgical Laboratory at University of Chicago where a tiny sample of curium was eventually separated and identified. The discovery was kept secret until after the end of World War II. The news was released to the public in November 1947. Most curium is produced by bombarding uranium or plutonium with neutrons in nuclear reactors – one tonne of spent nuclear fuel contains ~20 grams of curium.
Platinum bromide is the chemical compound with the formula PtBr2. This dark green powder is a common precursor to other platinum-bromide compounds. Like palladium chloride and palladium(II) bromide, it is a compound that dissolves only in coordinating solvents or in the presence of donor ligands.
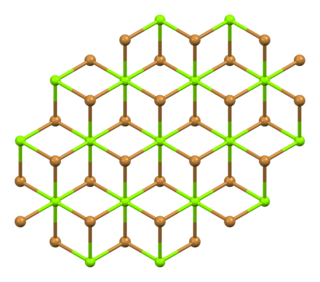
Magnesium bromide is a chemical compound of magnesium and bromine, with the chemical formula MgBr2. It is white and deliquescent crystalline solid. It is often used as a mild sedative and as an anticonvulsant for treatment of nervous disorders. It is water-soluble and somewhat soluble in alcohol. It can be found naturally in small amounts in some minerals such as: bischofite and carnallite, and in sea water, such as that of the Dead Sea.

Barium bromide is the chemical compound with the formula BaBr2. It is ionic and hygroscopic in nature.

Americium(III) chloride or americium trichloride is the chemical compound composed of americium and chlorine with the formula AmCl3. This salt forms pink hexagonal crystals. In the solid state each americium atom has nine chlorine atoms as near neighbours, at approximately the same distance, in a tricapped trigonal prismatic configuration.

Phenacyl bromide is the organic compound with the formula C6H5C(O)CH2Br. This colourless solid is a powerful lachrymator as well as a useful precursor to other organic compounds.

Americium(IV) fluoride is the inorganic compound with the formula AmF4. It is a tan solid. In terms of its structure, solid AmF4 features 8-coordinate Am centers interconnected by doubly bridging fluoride ligands.

Americium(III) fluoride or americium trifluoride is the chemical compound composed of americium and fluorine with the formula AmF3. It is a water soluble, pink salt.

Americium(III) bromide or americium tribromide is the chemical compound composed of americium and bromine with the formula AmBr3, with americium in a +3 oxidation state. The compound is a crystalline solid.
Americium(III) iodide or americium triiodide is the chemical compound, a salt composed of americium and iodine with the formula AmI3.

Americium(II) iodide is the inorganic compound with the formula AmI2. It is a black solid which crystallizes in the same motif as strontium bromide.
Americium bromide may refer to:
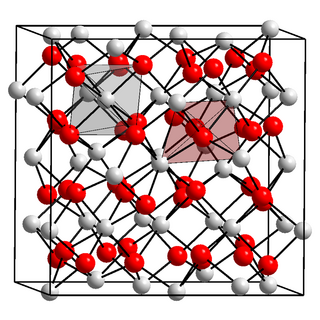
Americium(III) oxide or americium sesquioxide is an oxide of the element americium. It has the empirical formula Am2O3. Since all isotopes of americium are only artificially produced, americium (III) oxide has no natural occurrence. The colour depends on the crystal structure, of which there are more than one. It is soluble in acids.

Americium(III) hydroxide is a radioactive inorganic compound with the chemical formula Am(OH)3. It consists of one americium atom and three hydroxy groups. It was first discovered in 1944, closely related to the Manhattan Project. However, these results were confidential and were only released to the public in 1945. It was the first isolated sample of an americium compound, and the first americium compound discovered.
Americium(II) chloride, also known as dichloroamericium, is the chemical compound composed of americium and chloride with the formula AmCl2.

Americium(III) nitrate is an inorganic compound, a salt of americium and nitric acid with the chemical formula Am(NO3)3. The compound is soluble in water and radioactive.

Curium(III) bromide is the bromide salt of curium. It has an orthorhombic crystal structure.
Americium compounds are compounds containing the element americium (Am). These compounds can form in the +2, +3, and +4, although the +3 oxidation state is the most common. The +5, +6 and +7 oxidation states have also been reported.













