The actinide or actinoid series encompasses the 14 metallic chemical elements with atomic numbers from 89 to 103, actinium through Lawrencium. The actinide series derives its name from the first element in the series, actinium. The informal chemical symbol An is used in general discussions of actinide chemistry to refer to any actinide.

Bromine is a chemical element; it has symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig and Antoine Jérôme Balard, its name was derived from the Ancient Greek βρῶμος (bromos) meaning "stench", referring to its sharp and pungent smell.

Berkelium is a synthetic chemical element; it has symbol Bk and atomic number 97. It is a member of the actinide and transuranium element series. It is named after the city of Berkeley, California, the location of the Lawrence Berkeley National Laboratory where it was discovered in December 1949. Berkelium was the fifth transuranium element discovered after neptunium, plutonium, curium and americium.

Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion does not always result in fire, because a flame is only visible when substances undergoing combustion vaporize, but when it does, a flame is a characteristic indicator of the reaction. While activation energy must be supplied to initiate combustion, the heat from a flame may provide enough energy to make the reaction self-sustaining.

Protactinium is a chemical element; it has symbol Pa and atomic number 91. It is a dense, radioactive, silvery-gray actinide metal which readily reacts with oxygen, water vapor, and inorganic acids. It forms various chemical compounds, in which protactinium is usually present in the oxidation state +5, but it can also assume +4 and even +3 or +2 states. Concentrations of protactinium in the Earth's crust are typically a few parts per trillion, but may reach up to a few parts per million in some uraninite ore deposits. Because of its scarcity, high radioactivity, and high toxicity, there are currently no uses for protactinium outside scientific research, and for this purpose, protactinium is mostly extracted from spent nuclear fuel.

Thorium is a chemical element; it has symbol Th and atomic number 90. Thorium is a weakly radioactive light silver metal which tarnishes olive gray when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high melting point. Thorium is an electropositive actinide whose chemistry is dominated by the +4 oxidation state; it is quite reactive and can ignite in air when finely divided.
Sulfur trioxide (alternative spelling sulphur trioxide, also known as nisso sulfan) is the chemical compound with the formula SO3. It has been described as "unquestionably the most important economically" sulfur oxide. It is prepared on an industrial scale as a precursor to sulfuric acid.

Bismuth(III) oxide is perhaps the most industrially important compound of bismuth. It is also a common starting point for bismuth chemistry. It is found naturally as the mineral bismite (monoclinic) and sphaerobismoite, but it is usually obtained as a by-product of the smelting of copper and lead ores. Dibismuth trioxide is commonly used to produce the "Dragon's eggs" effect in fireworks, as a replacement of red lead.

Copper(II) bromide (CuBr2) is a chemical compound that forms an unstable tetrahydrate CuBr2·4H2O. It is used in photographic processing as an intensifier and as a brominating agent in organic synthesis.

Zirconium(IV) fluoride describes members of a family inorganic compounds with the formula (ZrF4(H2O)x. All are colorless, diamagnetic solids. Anhydrous Zirconium(IV) fluoride' is a component of ZBLAN fluoride glass.

Gallium(III) bromide (GaBr3) is a chemical compound, and one of four gallium trihalides.
Bromine compounds are compounds containing the element bromine (Br). These compounds usually form the -1, +1, +3 and +5 oxidation states. Bromine is intermediate in reactivity between chlorine and iodine, and is one of the most reactive elements. Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine. This can be seen from the standard electrode potentials of the X2/X− couples (F, +2.866 V; Cl, +1.395 V; Br, +1.087 V; I, +0.615 V; At, approximately +0.3 V). Bromination often leads to higher oxidation states than iodination but lower or equal oxidation states to chlorination. Bromine tends to react with compounds including M–M, M–H, or M–C bonds to form M–Br bonds.
Uranium hydride, also called uranium trihydride (UH3), is an inorganic compound and a hydride of uranium.

Compounds of lead exist with lead in two main oxidation states: +2 and +4. The former is more common. Inorganic lead(IV) compounds are typically strong oxidants or exist only in highly acidic solutions.
The tetrabromonickelate anion contains a doubly-charged nickel atom (Ni2+) surrounded by four bromide ions in a tetrahedral arrangement. The formula is [NiBr4]2−.

Many compounds of thorium are known: this is because thorium and uranium are the most stable and accessible actinides and are the only actinides that can be studied safely and legally in bulk in a normal laboratory. As such, they have the best-known chemistry of the actinides, along with that of plutonium, as the self-heating and radiation from them is not enough to cause radiolysis of chemical bonds as it is for the other actinides. While the later actinides from americium onwards are predominantly trivalent and behave more similarly to the corresponding lanthanides, as one would expect from periodic trends, the early actinides up to plutonium have relativistically destabilised and hence delocalised 5f and 6d electrons that participate in chemistry in a similar way to the early transition metals of group 3 through 8: thus, all their valence electrons can participate in chemical reactions, although this is not common for neptunium and plutonium.
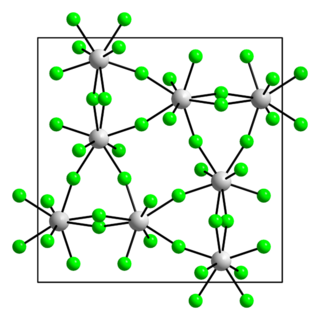
Protactinium(V) fluoride is a fluoride of protactinium with the chemical formula PaF5.

Protactinium(IV) bromide is an inorganic compound. It is an actinide halide, composed of protactinium and bromine. It is radioactive, and has the chemical formula of PaBr4. It may be due to the brown color of bromine that causes the appearance of protactinium(IV) bromide to be brown crystals. Its crystal structure is tetragonal. Protactinium(IV) bromide is sublimed in a vacuum at 400 °C. The protactinium(IV) halide closest in structure to protactinium(IV) bromide is protactinium(IV) chloride.
Protactinium compounds are compounds containing the element protactinium. These compounds usually have protactinium in the +5 oxidation state, although these compounds can also exist in the +2, +3 and +4 oxidation states.
Neptunium compounds are compounds containg the element neptunium (Np). Neptunium has five ionic oxidation states ranging from +3 to +7 when forming chemical compounds, which can be simultaneously observed in solutions. It is the heaviest actinide that can lose all its valence electrons in a stable compound. The most stable state in solution is +5, but the valence +4 is preferred in solid neptunium compounds. Neptunium metal is very reactive. Ions of neptunium are prone to hydrolysis and formation of coordination compounds.












