
The Montreal Protocol on Substances That Deplete the Ozone Layer is an international treaty designed to protect the ozone layer by phasing out the production of numerous substances that are responsible for ozone depletion. It was agreed on 16 September 1987, and entered into force on 1 January 1989. Since then, it has undergone several amendments and adjustments, with revisions agreed to in 1990 (London), 1992 (Copenhagen), 1995 (Vienna), 1997 (Montreal), 1999 (Beijing), 2007 (Montreal), 2016 (Kigali) and 2018 (Quito). As a result of the international agreement, the ozone hole in Antarctica is slowly recovering. Climate projections indicate that the ozone layer will return to 1980 levels between 2040 and 2066. Due to its widespread adoption and implementation, it has been hailed as an example of successful international co-operation. Former UN Secretary-General Kofi Annan stated that "perhaps the single most successful international agreement to date has been the Montreal Protocol". In comparison, effective burden-sharing and solution proposals mitigating regional conflicts of interest have been among the success factors for the ozone depletion challenge, where global regulation based on the Kyoto Protocol has failed to do so. In this case of the ozone depletion challenge, there was global regulation already being installed before a scientific consensus was established. Also, overall public opinion was convinced of possible imminent risks.

The ozone layer or ozone shield is a region of Earth's stratosphere that absorbs most of the Sun's ultraviolet radiation. It contains a high concentration of ozone (O3) in relation to other parts of the atmosphere, although still small in relation to other gases in the stratosphere. The ozone layer contains less than 10 parts per million of ozone, while the average ozone concentration in Earth's atmosphere as a whole is about 0.3 parts per million. The ozone layer is mainly found in the lower portion of the stratosphere, from approximately 15 to 35 kilometers (9 to 22 mi) above Earth, although its thickness varies seasonally and geographically.

Ozone depletion consists of two related events observed since the late 1970s: a steady lowering of about four percent in the total amount of ozone in Earth's atmosphere, and a much larger springtime decrease in stratospheric ozone around Earth's polar regions. The latter phenomenon is referred to as the ozone hole. There are also springtime polar tropospheric ozone depletion events in addition to these stratospheric events.

Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly halogenated hydrocarbons that contain carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F), produced as volatile derivatives of methane, ethane, and propane.
Dichlorodifluoromethane (R-12) is a colorless gas popularly known by the genericized brand name Freon. It is a chlorofluorocarbon halomethane (CFC) used as a refrigerant and aerosol spray propellant. In compliance with the Montreal Protocol, its manufacture was banned in developed countries in 1996, and in developing countries in 2010 out of concerns about its damaging effect on the ozone layer. Its only allowed usage is as a fire retardant in submarines and aircraft. It is soluble in many organic solvents. R-12 cylinders are colored white.
The ozone depletion potential (ODP) of a chemical compound is the relative amount of degradation to the ozone layer it can cause, with trichlorofluoromethane being fixed at an ODP of 1.0. Chlorodifluoromethane (R-22), for example, has an ODP of 0.05. CFC 11, or R-11 has the maximum potential amongst chlorocarbons because of the presence of three chlorine atoms in the molecule.
Trichlorofluoromethane, also called freon-11, CFC-11, or R-11, is a chlorofluorocarbon (CFC). It is a colorless, faintly ethereal, and sweetish-smelling liquid that boils around room temperature. CFC-11 is a Class 1 ozone-depleting substance which damages Earth's protective stratospheric ozone layer. R-11 is not flammable at ambient temperature and pressure but it can become very combustible if heated and ignited by a strong ignition source.
Fluoroform, or trifluoromethane, is the chemical compound with the formula CHF3. It is a hydrofluorocarbon as well as being a part of the haloforms, a class of compounds with the formula CHX3 with C3v symmetry. Fluoroform is used in diverse applications in organic synthesis. It is not an ozone depleter but is a greenhouse gas.
1,2-Dichlorotetrafluoroethane, or R-114, also known as cryofluorane (INN), is a chlorofluorocarbon (CFC) with the molecular formula ClF2CCF2Cl. Its primary use has been as a refrigerant. It is a non-flammable gas with a sweetish, chloroform-like odor with the critical point occurring at 145.6 °C and 3.26 MPa. When pressurized or cooled, it is a colorless liquid. It is listed on the Intergovernmental Panel on Climate Change's list of ozone depleting chemicals, and is classified as a Montreal Protocol Class I, group 1 ozone depleting substance.
Chlorotrifluoromethane, R-13, CFC-13, or Freon 13, is a non-flammable, non-corrosive, nontoxic chlorofluorocarbon (CFC) and also a mixed halomethane. It is a man-made substance used primarily as a refrigerant. When released into the environment, CFC-13 has a high ozone depletion potential, and long atmospheric lifetime. Only a few other greenhouse gases surpass CFC-13 in global warming potential (GWP). The IPCC AR5 reported that CFC-13's atmospheric lifetime was 640 years.
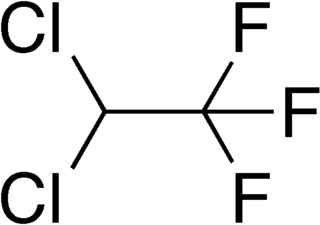
2,2-Dichloro-1,1,1-trifluoroethane or HCFC-123 is considered as an alternative to CFC-11 in low pressure refrigeration and HVAC systems, and should not be used in foam blowing processes or solvent applications. It is also the primary component of the Halotron I fire-extinguishing mixture.

1,1,1-Trifluoroethane, or R-143a or simply trifluoroethane, is a hydrofluorocarbon (HFC) compound that is a colorless gas. It should not be confused with the much more commonly used HFC gas R-134a, nor confused with the isomeric compound 1,1,2-trifluoroethane. 1,1,1-Trifluoroethane has a critical temperature of 73 °C.
The Vienna Convention for the Protection of the Ozone Layer is a multilateral environmental agreement signed in 1985 that provided frameworks for international reductions in the production of chlorofluorocarbons due to their contribution to the destruction of the ozone layer, resulting in an increased threat of skin cancer.
1,1,2-Trichloro-1,2,2-trifluoroethane, also called trichlorotrifluoroethane or CFC-113, is a chlorofluorocarbon. It has the formula Cl2FC−CClF2. This colorless, volatile liquid is a versatile solvent.
Ozone depletion and climate change are environmental challenges whose connections have been explored and which have been compared and contrasted, for example in terms of global regulation, in various studies and books.

The Kigali Amendment to the Montreal Protocol is an international agreement to gradually reduce the consumption and production of hydrofluorocarbons (HFCs). It is a legally binding agreement designed to create rights and obligations in international law.
Anne Ritger Douglass is atmospheric physicist known for her research on chlorinated compounds and the ozone layer.

1,3-Dichloro-1,1,2,2,3-pentafluoropropane is a hydrochlorofluorocarbon. It is a volatile derivative of propane which has served as an HCFC replacement for the CFC, 1,1,2-trichloro-1,2,2-trifluoroethane which was used as a cleaning agent which has been used in the aerospace and electronics industries since the phase out of class 1 ozone depleting substances by the Montreal Protocol. As of 2015 with the phase out of hydrochlorofluorocarbons, HCFC-225 is included in this phase out, and applications where it was used must now be fulfilled by non-ozone depleting substances.

Tetrachloro-1,1-difluoroethane or 1,1,1,2-tetrachloro-2,2-difluoroethane, Freon 112a, R-112a, or CFC-112a is an asymmetric chlorofluorocarbon isomer of tetrachloro-1,1-difluoroethane with formula CClF2CCl3. It contains ethane substituted by four chlorine atoms and two fluorine atoms. With a boiling point of 91.5°C it is the freon with second highest boiling point.

1,1-Dichlorotetrafluoroethane is a chlorofluorocarbon also known as CFC-114a or R114a by American Society of Heating, Refrigerating, and Air Conditioning Engineers. It has two chlorine atoms on one carbon atom and none on the other. It is one of two isomers of dichlorotetrafluoroethane, the other being 1,2-dichlorotetrafluoroethane, also known as CFC-114.











