
Benzimidazole is a heterocyclic aromatic organic compound. This bicyclic compound consists of the fusion of benzene and imidazole. It is a colorless solid.
Diphosphene is a type of organophosphorus compound that has a phosphorus-phosphorus double bond, denoted by R-P=P-R'. These compounds are not common but are of theoretical interest. Normally, compounds with the empirical formula RP exist as rings. However, like other multiple bonds between heavy main-group elements, P=P double bonds can be stabilized by a large steric hindrance from the substitutions. The first isolated diphosphene bis(2,4,6-tri-tert-butylphenyl)diphosphene was exemplified by Masaaki Yoshifuji and his coworkers in 1981, in which diphosphene is stabilized by two bulky phenyl group.
Organophosphorus compounds are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX nerve agents.
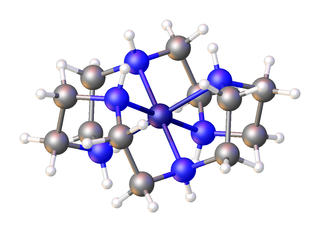
In chemistry, an aza-crown ether is an aza analogue of a crown ether (cyclic polyether). While the parent crown ethers have the formulae (CH2CH2O)n, the parent azacrown ethers have the formulae (CH2CH2NH)n, where n = 3, 4, 5, 6. Well studied aza crowns include triazacyclononane (n = 3), cyclen (n = 4), and hexaaza-18-crown-6 (n = 6).

Hapticity is the coordination of a ligand to a metal center via an uninterrupted and contiguous series of atoms. The hapticity of a ligand is described with the Greek letter η ('eta'). For example, η2 describes a ligand that coordinates through 2 contiguous atoms. In general the η-notation only applies when multiple atoms are coordinated. In addition, if the ligand coordinates through multiple atoms that are not contiguous then this is considered denticity, and the κ-notation is used once again. When naming complexes care should be taken not to confuse η with μ ('mu'), which relates to bridging ligands.

Iodosobenzene or iodosylbenzene is an organoiodine compound with the empirical formula C6H5IO. This colourless solid compound is used as an oxo transfer reagent in research laboratories examining organic and coordination chemistry.

1,4,7-Trithiacyclononane, also called 9-ane-S3, is the heterocyclic compound with the formula (CH2CH2S)3. This cyclic thioether is most often encountered as a tridentate ligand in coordination chemistry.

1,2-Bis(dimethylarsino)benzene (diars) is the organoarsenic compound with the formula C6H4(As(CH3)2)2. The molecule consists of two dimethylarsino groups attached to adjacent carbon centers of a benzene ring. It is a chelating ligand in coordination chemistry. This colourless oil is commonly abbreviated "diars."
Organoarsenic chemistry is the chemistry of compounds containing a chemical bond between arsenic and carbon. A few organoarsenic compounds, also called "organoarsenicals," are produced industrially with uses as insecticides, herbicides, and fungicides. In general these applications are declining in step with growing concerns about their impact on the environment and human health. The parent compounds are arsine and arsenic acid. Despite their toxicity, organoarsenic biomolecules are well known.

Diphosphines, sometimes called bisphosphanes, are organophosphorus compounds most commonly used as bidentate phosphine ligands in inorganic and organometallic chemistry. They are identified by the presence of two phosphino groups linked by a backbone, and are usually chelating. A wide variety of diphosphines have been synthesized with different linkers and R-groups. Alteration of the linker and R-groups alters the electronic and steric properties of the ligands which can result in different coordination geometries and catalytic behavior in homogeneous catalysts.

Tris(ethylenediamine)cobalt(III) chloride is an inorganic compound with the formula [Co(en)3]Cl3 (where "en" is the abbreviation for ethylenediamine). It is the chloride salt of the coordination complex [Co(en)3]3+. This trication was important in the history of coordination chemistry because of its stability and its stereochemistry. Many different salts have been described. The complex was first described by Alfred Werner who isolated this salt as yellow-gold needle-like crystals.

In organometallic chemistry, a metallacycle is a derivative of a carbocyclic compound wherein a metal has replaced at least one carbon center; this is to some extent similar to heterocycles. Metallacycles appear frequently as reactive intermediates in catalysis, e.g. olefin metathesis and alkyne trimerization. In organic synthesis, directed ortho metalation is widely used for the functionalization of arene rings via C-H activation. One main effect that metallic atom substitution on a cyclic carbon compound is distorting the geometry due to the large size of typical metals.

1,1-Bis(diphenylphosphino)methane (dppm), is an organophosphorus compound with the formula CH2(PPh2)2. Dppm, a white, crystalline powder, is used in inorganic and organometallic chemistry as a ligand. It is more specifically a chelating ligand because it is a ligand that can bond to metals with two phosphorus donor atoms. The natural bite angle is 73°.

DOTA (also known as tetraxetan) is an organic compound with the formula (CH2CH2NCH2CO2H)4. The molecule consists of a central 12-membered tetraaza (i.e., containing four nitrogen atoms) ring. DOTA is used as a complexing agent, especially for lanthanide ions. Its complexes have medical applications as contrast agents and cancer treatments.

Cyclam (1,4,8,11-tetraazacyclotetradecane) is an organic compound with the formula (NHCH2CH2NHCH2CH2CH2)2. Classified as an aza-crown ether, it is a white solid that is soluble in water. As a macrocyclic ligand, it binds strongly to many transition metal cations. The compound was first prepared by the reaction of 1,3-dibromopropane and ethylenediamine.
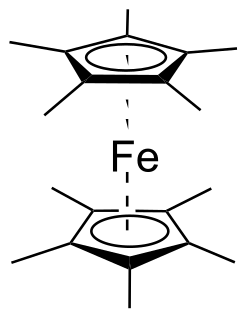
Decamethylferrocene or bis(pentamethylcyclopentadienyl)iron(II) is a chemical compound with formula Fe(C
5(CH
3)
5)
2 or C
20H
30Fe. It is a sandwich compound, whose molecule has an iron(II) cation Fe2+ attached by coordination bonds between two pentamethylcyclopentadienyl anions (Cp*−, (CH
3)
5C−
5). It can also be viewed as a derivative of ferrocene, with a methyl group replacing each hydrogen atom of its cyclopentadienyl rings. The name and formula are often abbreviated to DmFc, Me
10Fc or FeCp*
2.

Half sandwich compounds are organometallic complexes that feature a cyclic polyhapto ligand bound to an MLn center, where L is a unidentate ligand. Thousands of such complexes are known. Well-known examples include cyclobutadieneiron tricarbonyl and (C5H5)TiCl3. Commercially useful examples include (C5H5)Co(CO)2, which is used in the synthesis of substituted pyridines, and methylcyclopentadienyl manganese tricarbonyl, an antiknock agent in petrol.
Organoniobium chemistry is the chemistry of compounds containing niobium-carbon (Nb-C) bonds. Compared to the other group 5 transition metal organometallics, the chemistry of organoniobium compounds most closely resembles that of organotantalum compounds. Organoniobium compounds of oxidation states +5, +4, +3, +2, +1, 0, -1, and -3 have been prepared, with the +5 oxidation state being the most common.
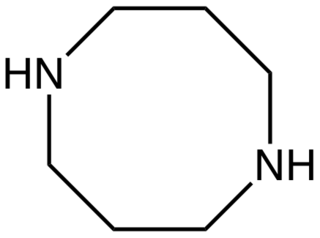
1,5-Diazacyclooctane is an organic compound with the formula (CH2CH2CH2NH)2. It is a colorless oil. 1,5-Diazacyclooctane is a cyclic diamine.

Transition metal thioether complexes comprise coordination complexes of thioether (R2S) ligands. The inventory is extensive.

















