
A pheromone is a secreted or excreted chemical factor that triggers a social response in members of the same species. Pheromones are chemicals capable of acting like hormones outside the body of the secreting individual, to affect the behavior of the receiving individuals. There are alarm pheromones, food trail pheromones, sex pheromones, and many others that affect behavior or physiology. Pheromones are used by many organisms, from basic unicellular prokaryotes to complex multicellular eukaryotes. Their use among insects has been particularly well documented. In addition, some vertebrates, plants and ciliates communicate by using pheromones. The ecological functions and evolution of pheromones are a major topic of research in the field of chemical ecology.
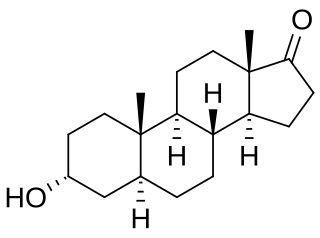
Androsterone, or 3α-hydroxy-5α-androstan-17-one, is an endogenous steroid hormone, neurosteroid, and putative pheromone. It is a weak androgen with a potency that is approximately 1/7 that of testosterone. Androsterone is a metabolite of testosterone and dihydrotestosterone (DHT). In addition, it can be converted back into DHT via 3α-hydroxysteroid dehydrogenase and 17β-hydroxysteroid dehydrogenase, bypassing conventional intermediates such as androstanedione and testosterone, and as such, can be considered to be a metabolic intermediate in its own right.
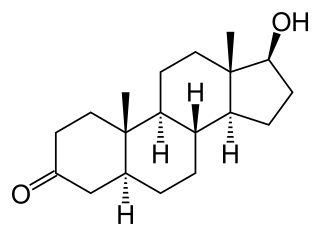
Dihydrotestosterone is an endogenous androgen sex steroid and hormone primarily involved in the growth and repair of the prostate, the production of sebum, and body hair composition.
Body odor or body odour (BO) is present in all animals and its intensity can be influenced by many factors. Body odor has a strong genetic basis, but can also be strongly influenced by various factors, such as gender, diet, health, and medication. The body odor of human males plays an important role in human sexual attraction, as a powerful indicator of MHC/HLA heterozygosity. Significant evidence suggests that women are attracted to men whose body odor is different from theirs, indicating that they have immune genes that are different from their own, which may produce healthier offspring.

Androstenone (5α-androst-16-en-3-one) is a 16-androstene class steroidal pheromone. It is found in boar's saliva, celery cytoplasm, and truffle fungus. Androstenone was the first mammalian pheromone to be identified. It is found in high concentrations in the saliva of male pigs, and, when inhaled by a female pig that is in heat, results in the female assuming the mating stance. Androstenone is the active ingredient in 'Boarmate', a commercial product made by DuPont sold to pig farmers to test sows for timing of artificial insemination.

Metandienone, also known as methandienone or methandrostenolone and sold under the brand name Dianabol (D-Bol) among others, is an androgen and anabolic steroid (AAS) medication which is still quite often used because of its affordability and effectiveness for bulking cycles. It is also used non-medically for physique- and performance-enhancing purposes. It is often taken by mouth.

Androstenol, also known as 5α-androst-16-en-3α-ol, is a 16-androstene class steroidal pheromone and neurosteroid in humans and other mammals, notably pigs. It possesses a characteristic musk-like odor.

Androstadienone, or androsta-4,16-dien-3-one, is a 16-androstene class endogenous steroid that has been described as having potent pheromone-like activities in humans. The compound is synthesized from androstadienol by 3β-hydroxysteroid dehydrogenase, and can be converted into androstenone by 5α-reductase, which can subsequently be converted into 3α-androstenol or 3β-androstenol by 3-ketosteroid reductase.

Epiandrosterone, or isoandrosterone, also known as 3β-androsterone, 3β-hydroxy-5α-androstan-17-one, or 5α-androstan-3β-ol-17-one, is a steroid hormone with weak androgenic activity. It is a metabolite of testosterone and dihydrotestosterone (DHT). It was first isolated in 1931, by Adolf Friedrich Johann Butenandt and Kurt Tscherning. They distilled over 17,000 litres of male urine, from which they got 50 milligrams of crystalline androsterone, which was sufficient to find that the chemical formula was very similar to estrone.

5α-Androst-2-en-17-one is an endogenous, naturally occurring, orally active anabolic-androgenic steroid (AAS) and a derivative of dihydrotestosterone (DHT). It is a metabolite of dehydroepiandrosterone (DHEA) in the body and is also a pheromone found in elephants and boars. 5α-Androst-2-en-17-one has been sold on the Internet as a "dietary supplement". It resembles desoxymethyltestosterone (17α-methyl-5α-androst-2-en-17β-ol) in chemical structure and may act as an androgen prohormone.

3β-Androstanediol, also known as 5α-androstane-3β,17β-diol, and sometimes shortened in the literature to 3β-diol, is an endogenous steroid hormone and a metabolite of androgens like dehydroepiandrosterone (DHEA) and dihydrotestosterone (DHT).

Androstadienol, or androsta-5,16-dien-3β-ol, is a 16-androstene class endogenous steroid, pheromone, and chemical intermediate to several other pheromones that is found in the sweat of both men and women.
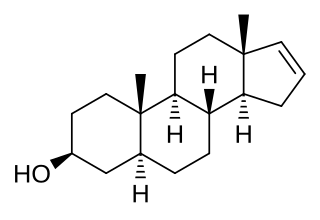
3β-Androstenol, also known as 5α-androst-16-en-3β-ol, is a naturally occurring mammalian pheromone known to be present in humans and pigs. It is thought to play a role in axillary odor. It is produced from androstenone via the enzyme 3β-hydroxysteroid dehydrogenase. Unlike its C3α epimer 3α-androstenol, 3β-androstenol shows no potentiation of the GABAA receptor or anticonvulsant activity.
A xenobiotic-sensing receptor is a receptor that binds xenobiotics. They include the following nuclear receptors:
No study has led to the isolation of true human sex pheromones, though various researchers have investigated the possibility of their existence. Sex pheromones are chemical (olfactory) signals, pheromones, released by an organism to attract an individual, encourage it to mate with it, or perform some other function closely related with sexual reproduction. While humans are highly dependent upon visual cues, when in proximity, smells also play a role in sociosexual behaviors. An inherent difficulty in studying human pheromones is the need for cleanliness and odorlessness in human participants. Experiments have focused on three classes of putative human pheromones: axillary steroids, vaginal aliphatic acids, and stimulators of the vomeronasal organ.
The biochemistry of body odor pertains to the chemical compounds in the body responsible for body odor and their kinetics.
The androgen backdoor pathway is a collective name for all metabolic pathways where clinically relevant androgens are synthesized from 21-carbon steroids (pregnanes) by their 5α-reduction, bypassing testosterone and/or androstenedione.

5α-Pregnan-17α-ol-3,20-dione, also known as 17α-hydroxy-dihydroprogesterone (17‐OH-DHP) is an endogenous steroid, a metabolite of 17α-hydroxyprogesterone.












