
Anthracene is a solid polycyclic aromatic hydrocarbon (PAH) of formula C14H10, consisting of three fused benzene rings. It is a component of coal tar. Anthracene is used in the production of the red dye alizarin and other dyes. Anthracene is colorless but exhibits a blue (400–500 nm peak) fluorescence under ultraviolet radiation.

Phenanthrene a polycyclic aromatic hydrocarbon (PAH) with formula C14H10, consisting of three fused benzene rings. It is a colorless, crystal-like solid, but can also appear yellow. Phenanthrene is used to make dyes, plastics and pesticides, explosives and drugs. It has also been used to make bile acids, cholesterol and steroids.

Fichtelite is a rare white mineral found in fossilized wood from Bavaria. It crystallizes in the monoclinic crystal system. It is a cyclic hydrocarbon: dimethyl-isopropyl-perhydrophenanthrene, C19H34. It is very soft with a Mohs hardness of 1, the same as talc. Its specific gravity is very low at 1.032, just slightly denser than water.

Morphinan is the prototype chemical structure of a large chemical class of psychoactive drugs, consisting of opiate analgesics, cough suppressants, and dissociative hallucinogens, among others.

Phenanthrenoids are chemical compounds formed with a phenanthrene backbone. These compounds occur naturally in plants, although they can also be synthesized.

Chrysene is a polycyclic aromatic hydrocarbon (PAH) with the molecular formula C
18H
12 that consists of four fused benzene rings. It is a natural constituent of coal tar, from which it was first isolated and characterized. It is also found in creosote at levels of 0.5-6 mg/kg.

Coelogyne cristata is an epiphytic orchid that comes from cool, moist areas of the eastern Himalayas and Vietnam. It blooms every spring, before the snow begins to melt. Its genus name Coelogyne originates from two Greek words, koilos (“hollow”) and gyne (“woman”), because of the orchid’s pistil. Cristata takes its species name from crista, the Latin word for “comb”, because of the look of the flower’s lip.
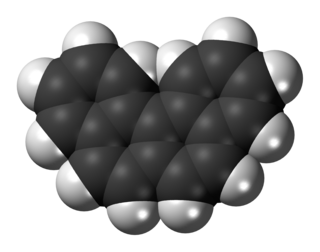
Benzo[c]phenanthrene is a polycyclic aromatic hydrocarbon with the chemical formula C18H12. It is a white solid that is soluble in nonpolar organic solvents. It is a nonplanar molecule consisting of the fusion of four fused benzene rings. The compound is of mainly theoretical interest but it is environmentally occurring and weakly carcinogenic.
Bulbophyllum reptans is a species of orchid in the genus Bulbophyllum.

Gonane is a chemical compound with formula C
17H
28, whose molecule can be described as three molecules of cyclohexane and one of cyclopentane, fused in a particular way. More specifically, the molecule can be described as that of cycloheptadecane (–CH2–)17 with three extra bonds connecting carbons 1 to 13, 4 to 12, and 5 to 9, replacing six hydrogen atoms. It can also be viewed as the result of fusing a cyclopentane molecule with a fully hydrogenated molecule of phenanthrene, hence the more descriptive name perhydrocyclopenta[a]phenanthrene.

In organic chemistry, the Mallory reaction is a photochemical-cyclization–elimination reaction of diaryl-ethylene structures to form phenanthrenes and other polycyclic form polycyclic aromatic hydrocarbons and heteroaromatics. This name reaction is named for Frank Mallory, who discovered it while a graduate student.

1-Hydroxyphenanthrene is a phenanthrol and a human metabolite of phenanthrene that can be detected in urine of persons exposed to PAHs.

Nudol is a phenanthrenoid of the orchids Eulophia nuda, Eria carinata, Eria stricta and Maxillaria densa.
Cirrhopetalum maculosum is an orchid species in the genus Cirrhopetalum.
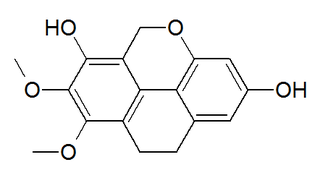
Coelogin is a phenanthrenoid found in the high altitude Himalayan orchid Coelogyne cristata. This molecule has a phenanthro[4,5-bcd]pyran structure.
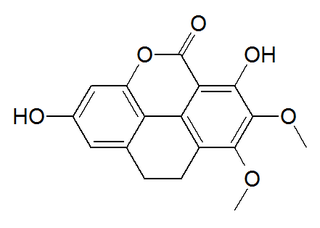
Coeloginin is a phenanthrenoid found in the high altitude Himalayan orchid Coelogyne cristata. This molecule has a phenanthro[4,5-bcd]pyrone structure.

Coeloginanthrin is a phenanthrenoid found in the orchid Coelogyne cristata.
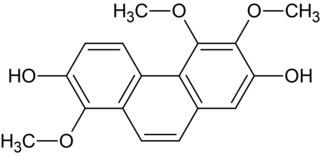
Confusarin is a phenanthrenoid found in the orchids Eria confusa and Bulbophyllum reptans. It can also be synthesized.

Triphenylethylene (TPE) is a simple aromatic hydrocarbon that possesses weak estrogenic activity. Its estrogenic effects were discovered in 1937. TPE was derived from structural modification of the more potent estrogen diethylstilbestrol, which is a member of the stilbestrol group of nonsteroidal estrogens.

Phenanthrenedione is a quinone derivative of a polycyclic aromatic hydrocarbon. It is an orange, water-insoluble solid.


















