| |||||
| Decades: | |||||
|---|---|---|---|---|---|
| See also: | |||||
Events from the year 1979 in Jordan .
| |||||
| Decades: | |||||
|---|---|---|---|---|---|
| See also: | |||||
Events from the year 1979 in Jordan .
| | This section is empty. You can help by adding to it. (January 2015) |

The Moon is Earth's only natural satellite. At about one-quarter the diameter of Earth, it is the largest natural satellite in the Solar System relative to the size of a major planet, the fifth largest satellite in the Solar System overall, and larger than any known dwarf planet. The Moon is a planetary-mass object that formed a differentiated rocky body, making it a satellite planet under the geophysical definitions of the term. It lacks any significant atmosphere, hydrosphere, or magnetic field. Its surface gravity is about one-sixth of Earth's ; Jupiter's moon Io is the only satellite in the Solar System known to have a higher surface gravity and density.

Blackburn Rovers Football Club is a professional football club, based in Blackburn, Lancashire, England, which competes in the EFL Championship, the second tier of the English football league system.

Ville is a commune in the Oise department in the region of Hauts-de-France in northern France. The village is divided into a northern part and a southern part by the Divette river.

The Marvel Cinematic Universe (MCU) is an American media franchise and shared universe centered on a series of superhero films produced by Marvel Studios. The films are based on characters that appear in American comic books published by Marvel Comics. The franchise also includes television series, short films, digital series, and literature. The shared universe, much like the original Marvel Universe in comic books, was established by crossing over common plot elements, settings, cast, and characters.

The COVID-19 pandemic, also known as the coronavirus pandemic, is an ongoing global pandemic of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The novel virus was first identified from an outbreak in the Chinese city of Wuhan in December 2019, and attempts to contain it there failed, allowing it to spread across the globe. The World Health Organization (WHO) declared a Public Health Emergency of International Concern on 30 January 2020 and a pandemic on 11 March 2020. As of 21 January 2022, the pandemic had caused more than 343 million cases and 5.57 million deaths, making it one of the deadliest in history.

Coronavirus disease 2019 (COVID-19) is a contagious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The first known case was identified in Wuhan, China, in December 2019. The disease has since spread worldwide, leading to an ongoing pandemic.

The COVID-19 pandemic in the United States is part of the worldwide pandemic of coronavirus disease 2019. Since January 2020, 69,308,111 confirmed cases have been reported with 860,247 deaths, the most of any country, and the nineteenth-highest per capita worldwide. As many infections have gone undetected, the Centers for Disease Control and Prevention (CDC) estimated that, as of May 2021, there could be a total 120.2 million infections in the United States, or more than a third of the total population. COVID-19 is the deadliest pandemic in U.S. history; it was the third-leading cause of death in the U.S. in 2020, behind heart disease and cancer. From 2019 to 2020, U.S. life expectancy dropped by 3 years for Hispanic Americans, 2.9 years for African Americans, and 1.2 years for white Americans. These effects have persisted as U.S. deaths due to COVID-19 in 2021 exceeded those in 2020.

An mRNAvaccine is a type of vaccine that uses a copy of a molecule called messenger RNA (mRNA) to produce an immune response. The vaccine delivers molecules of antigen-encoding mRNA into immune cells, which use the designed mRNA as a blueprint to build foreign protein that would normally be produced by a pathogen or by a cancer cell. These protein molecules stimulate an adaptive immune response that teaches the body to identify and destroy the corresponding pathogen or cancer cells. The mRNA is delivered by a co-formulation of the RNA encapsulated in lipid nanoparticles that protect the RNA strands and help their absorption into the cells.

A COVID‑19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2), the virus that causes coronavirus disease 2019 (COVID‑19).

The Moderna COVID‑19 vaccine, codenamed mRNA-1273 and sold under the brand name Spikevax, is a COVID-19 vaccine developed by American company Moderna, the United States National Institute of Allergy and Infectious Diseases (NIAID), and the Biomedical Advanced Research and Development Authority (BARDA). It is authorized for use in people aged twelve years and older in some jurisdictions and for people eighteen years and older in other jurisdictions to provide protection against COVID-19 which is caused by infection by the SARS-CoV-2 virus. It is designed to be administered as two or three 0.5 mL doses given by intramuscular injection at an interval of at least 28 days apart.

Molnupiravir is an antiviral medication that inhibits the replication of certain RNA viruses. It is used to treat COVID-19 in those infected by SARS-CoV-2.
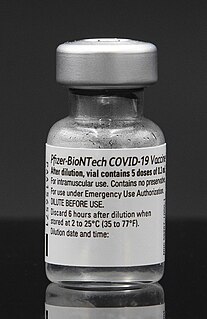
The Pfizer–BioNTech COVID-19 vaccine, sold under the brand name Comirnaty, is an mRNA-based COVID-19 vaccine developed by the German biotechnology company BioNTech and for its development collaborated with American company Pfizer, for support with clinical trials, logistics, and manufacturing. It is authorized for use in people aged five years and older in some jurisdictions, twelve years and older in some jurisdictions, and for people sixteen years and older in other jurisdictions, to provide protection against COVID-19, caused by infection with the SARS-CoV-2 virus. The vaccine is given by intramuscular injection. It is composed of nucleoside-modified mRNA (modRNA) encoding a mutated form of the full-length spike protein of SARS-CoV-2, which is encapsulated in lipid nanoparticles. Initial advice indicated that vaccination required two doses given 21 days apart, but the interval was later extended to up to 42 days in the US, and up to four months in Canada.

There are many variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19). Some are believed, or have been stated, to be of particular importance due to their potential for increased transmissibility, increased virulence, or reduced effectiveness of vaccines against them. These variants contribute to the continuation of the COVID-19 pandemic.

ZF2001, trade-named Zifivax or ZF-UZ-VAC-2001, is an adjuvanted protein subunit COVID-19 vaccine developed by Anhui Zhifei Longcom in collaboration with the Institute of Microbiology at the Chinese Academy of Sciences. The vaccine candidate is in Phase III trials with 29,000 participants in China, Ecuador, Malaysia, Pakistan, and Uzbekistan.

Corbevax is a protein subunit COVID-19 vaccine developed by Texas Children's Hospital Center for Vaccine Development and Baylor College of Medicine in Houston, Texas and Dynavax technologies based in Emeryville California. It is licensed to Indian biopharmaceutical firm Biological E. Limited (BioE) for development and production.
Sotrovimab, sold under the brand name Xevudy, is a human neutralizing monoclonal antibody with activity against severe acute respiratory syndrome coronavirus 2, known as SARS-CoV-2. It is under development by GlaxoSmithKline and Vir Biotechnology, Inc. Sotrovimab is designed to attach to the spike protein of SARS-CoV-2.

GBP510 is a COVID-19 vaccine candidate developed by SK Bioscience and GSK.

COVID-19 vaccine clinical research uses clinical research to establish the characteristics of COVID-19 vaccines. These characteristics include efficacy, effectiveness and safety. Thirty vaccines are authorized for use by national governments, including eight approved for emergency or full use by at least one WHO-recognised stringent regulatory authority; while five are in Phase IV. 204 vaccines are undergoing clinical trials that have yet to be authorized. Nine clinical trials consider heterologous vaccination courses.

The Omicron variant (B.1.1.529) is a variant of SARS-CoV-2 that was first reported to the World Health Organization (WHO) from South Africa on 24 November 2021.

SCTV01C is a COVID-19 vaccine candidate developed by Sinocelltech.