Antioxidants are compounds that inhibit oxidation, a chemical reaction that can produce free radicals. Autoxidation leads to degradation of organic compounds, including living matter. Antioxidants are frequently added to industrial products, such as polymers, fuels, and lubricants, to extend their usable lifetimes. Foods are also treated with antioxidants to forestall spoilage, in particular the rancidification of oils and fats. In cells, antioxidants such as glutathione, mycothiol, or bacillithiol, and enzyme systems like superoxide dismutase, can prevent damage from oxidative stress.

In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups bonded directly to an aromatic hydrocarbon group. The simplest is phenol, C
6H
5OH. Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule.

Butylated hydroxytoluene (BHT), also known as dibutylhydroxytoluene, is a lipophilic organic compound, chemically a derivative of phenol, that is useful for its antioxidant properties. BHT is widely used to prevent free radical-mediated oxidation in fluids and other materials, and the regulations overseen by the U.S. F.D.A.—which considers BHT to be "generally recognized as safe"—allow small amounts to be added to foods. Despite this, and the earlier determination by the National Cancer Institute that BHT was noncarcinogenic in an animal model, societal concerns over its broad use have been expressed. BHT has also been postulated as an antiviral drug, but as of December 2022, use of BHT as a drug is not supported by the scientific literature and it has not been approved by any drug regulatory agency for use as an antiviral.

Polyphenols are a large family of naturally occurring phenols. They are abundant in plants and structurally diverse. Polyphenols include phenolic acids, flavonoids, tannic acid, and ellagitannin, some of which have been used historically as dyes and for tanning garments.

In chemistry and biology, reactive oxygen species (ROS) are highly reactive chemicals formed from diatomic oxygen (O2), water, and hydrogen peroxide. Some prominent ROS are hydroperoxide (O2H), superoxide (O2-), hydroxyl radical (OH.), and singlet oxygen. ROS are pervasive because they are readily produced from O2, which is abundant. ROS are important in many ways, both beneficial and otherwise. ROS function as signals, that turn on and off biological functions. They are intermediates in the redox behavior of O2, which is central to fuel cells. ROS are central to the photodegradation of organic pollutants in the atmosphere. Most often however, ROS are discussed in a biological context, ranging from their effects on aging and their role in causing dangerous genetic mutations.

Tetrahydrobiopterin (BH4, THB), also known as sapropterin (INN), is a cofactor of the three aromatic amino acid hydroxylase enzymes, used in the degradation of amino acid phenylalanine and in the biosynthesis of the neurotransmitters serotonin (5-hydroxytryptamine, 5-HT), melatonin, dopamine, norepinephrine (noradrenaline), epinephrine (adrenaline), and is a cofactor for the production of nitric oxide (NO) by the nitric oxide synthases. Chemically, its structure is that of a (dihydropteridine reductase) reduced pteridine derivative (quinonoid dihydrobiopterin).

Synephrine, or, more specifically, p-synephrine, is an alkaloid, occurring naturally in some plants and animals, and also in approved drugs products as its m-substituted analog known as neo-synephrine. p-Synephrine and m-synephrine are known for their longer acting adrenergic effects compared to epinephrine and norepinephrine. This substance is present at very low concentrations in common foodstuffs such as orange juice and other orange products, both of the "sweet" and "bitter" variety. The preparations used in traditional Chinese medicine (TCM), also known as Zhi Shi (枳实), are the immature and dried whole oranges from Citrus aurantium. Extracts of the same material or purified synephrine are also marketed in the US, sometimes in combination with caffeine, as a weight-loss-promoting dietary supplement for oral consumption. While the traditional preparations have been in use for millennia as a component of TCM-formulas, synephrine itself is not an approved over the counter drug. As a pharmaceutical, m-synephrine (phenylephrine) is still used as a sympathomimetic, mostly by injection for the treatment of emergencies such as shock, and rarely orally for the treatment of bronchial problems associated with asthma and hay-fever.
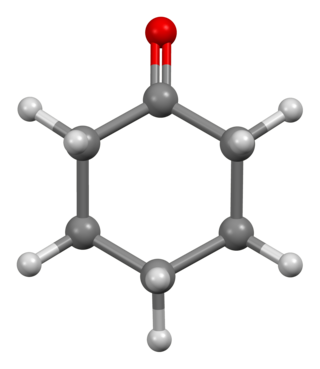
Cyclohexanone is the organic compound with the formula (CH2)5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oily liquid has a sweet odor reminiscent of benzaldehyde. Over time, samples of cyclohexanone assume a pale yellow color.

Oxidative stress reflects an imbalance between the systemic manifestation of reactive oxygen species and a biological system's ability to readily detoxify the reactive intermediates or to repair the resulting damage. Disturbances in the normal redox state of cells can cause toxic effects through the production of peroxides and free radicals that damage all components of the cell, including proteins, lipids, and DNA. Oxidative stress from oxidative metabolism causes base damage, as well as strand breaks in DNA. Base damage is mostly indirect and caused by the reactive oxygen species generated, e.g., O−
2, OH and H2O2. Further, some reactive oxidative species act as cellular messengers in redox signaling. Thus, oxidative stress can cause disruptions in normal mechanisms of cellular signaling.

Neuroprotection refers to the relative preservation of neuronal structure and/or function. In the case of an ongoing insult the relative preservation of neuronal integrity implies a reduction in the rate of neuronal loss over time, which can be expressed as a differential equation.

Caffeic acid is an organic compound with the formula (HO)2C6H3CH=CHCO2H. It is a polyphenol. It is a yellow solid. Structurally, it is classified as a hydroxycinnamic acid. The molecule consists of both phenolic and acrylic functional groups. It is found in all plants as an intermediate in the biosynthesis of lignin, one of the principal components of biomass and its residues. It is chemically unrelated to caffeine; the related name is due to its presence in coffee.
Oxygen radical absorbance capacity (ORAC) was a method of measuring antioxidant capacities in biological samples in vitro. Because no physiological proof in vivo existed in support of the free-radical theory or that ORAC provided information relevant to biological antioxidant potential, it was withdrawn in 2012.

Dirigent proteins are members of a class of proteins which dictate the stereochemistry of a compound synthesized by other enzymes. The first dirigent protein was discovered in Forsythia intermedia. This protein has been found to direct the stereoselective biosynthesis of (+)-pinoresinol from coniferyl alcohol monomers:

A polyphenol antioxidant is a hypothesized type of antioxidant studied in vitro. Numbering over 4,000 distinct chemical structures mostly from plants, such polyphenols have not been demonstrated to be antioxidants in vivo.
Chemical antagonists impede the normal function of a system. They function to invert the effects of other molecules. The effects of antagonists can be seen after they have encountered an agonist, and as a result, the effects of the agonist is neutralized. Antagonists such as dopamine antagonist slow down movement in lab rats. Although they hinder the joining of enzymes to substrates, Antagonists can be beneficial. For example, not only do angiotensin receptor blockers, and angiotensin-converting enzyme (ACE) inhibitors work to lower blood pressure, but they also counter the effects of renal disease in diabetic and non-diabetic patients. Chelating agents, such as calcium di sodium defeated, fall into the category of antagonists and operate to minimize the lethal effects of heavy metals such as mercury or lead.

The Folin–Ciocâlteu reagent (FCR) or Folin's phenol reagent or Folin–Denis reagent, is a mixture of phosphomolybdate and phosphotungstate used for the colorimetric in vitro assay of phenolic and polyphenolic antioxidants, also called the gallic acid equivalence method (GAE). It is named after Otto Folin, Vintilă Ciocâlteu, and Willey Glover Denis. The Folin-Denis reagent is prepared by mixing sodium tungstate and phosphomolybdic acid in phosphoric acid. The Folin–Ciocalteu reagent is just a modification of the Folin-Denis reagent. The modification consisted of the addition of lithium sulfate and bromine to the phosphotungstic-phosphomolybdic reagent.

Cystamine (2,2'-dithiobisethanamine) is an organic disulfide. It is formed when cystine is heated, the result of decarboxylation. Cystamine is an unstable liquid and is generally handled as the dihydrochloride salt, C4H12N2S2·2HCl, which is stable to 203-214 °C at which point it decomposes. Cystamine is toxic if swallowed or inhaled and potentially harmful by contact.

Dichlorofluorescein (DCF) is an organic dye of the fluorescein family, being substituted at the 2 and 7 positions by chloride.

In biochemistry, naturally occurring phenols are natural products containing at least one phenol functional group. Phenolic compounds are produced by plants and microorganisms. Organisms sometimes synthesize phenolic compounds in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research. Some phenols are germicidal and are used in formulating disinfectants.
Antioxidative stress is an overabundance of bioavailable antioxidant compounds that interfere with the immune system's ability to neutralize pathogenic threats. The fundamental opposite is oxidative stress, which can lead to such disease states as coronary heart disease or cancer.