| |||
| Names | |||
|---|---|---|---|
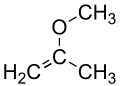
| Preferred IUPAC name 2-Methoxyprop-1-ene | |||
| Other names Methyl isopropenyl ether Isopropenyl methyl ether | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.751 | ||
PubChem CID | |||
| UNII | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| C4H8O | |||
| Molar mass | 72.107 g·mol−1 | ||
| Appearance | Colorless liquid [1] | ||
| Density | 0.753 g/mL [2] | ||
| Boiling point | 34 to 36 °C (93 to 97 °F; 307 to 309 K) [2] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
2-Methoxypropene is an ether with the chemical formula C4H8O. It is a reagent used in organic synthesis to introduce a protecting group for alcohols, [1] and the conversion diols to the acetonide group. [3]
2-Methoxypropene can be prepared by the elimination of methanol from dimethoxypropane, [4] or by the addition of methanol to propyne or allene. [5]