A lubricant is a substance that helps to reduce friction between surfaces in mutual contact, which ultimately reduces the heat generated when the surfaces move. It may also have the function of transmitting forces, transporting foreign particles, or heating or cooling the surfaces. The property of reducing friction is known as lubricity.

In organic chemistry, a thiol, or thiol derivative, is any organosulfur compound of the form R−SH, where R represents an alkyl or other organic substituent. The −SH functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. Thiols are the sulfur analogue of alcohols, and the word is a blend of "thio-" with "alcohol".

Methanethiol is an organosulfur compound with the chemical formula CH
3SH. It is a colorless gas with a distinctive putrid smell. It is a natural substance found in the blood, brain and feces of animals, as well as in plant tissues. It also occurs naturally in certain foods, such as some nuts and cheese. It is one of the chemical compounds responsible for bad breath and the smell of flatus. Methanethiol is the simplest thiol and is sometimes abbreviated as MeSH. It is very flammable.
Sulfide (also sulphide in British English ) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. Sulfide also refers to large families of inorganic and organic compounds, e.g. lead sulfide and dimethyl sulfide. Hydrogen sulfide (H2S) and bisulfide (SH−) are the conjugate acids of sulfide.

Synthetic oil is a lubricant consisting of chemical compounds that are artificially modified or synthesised. Synthetic lubricants can be manufactured using chemically modified petroleum components rather than whole crude oil, but can also be synthesized from other raw materials. The base material, however, is still overwhelmingly crude oil that is distilled and then modified physically and chemically. The actual synthesis process and composition of additives is generally a commercial trade secret and will vary among producers.

Ethanethiol, commonly known as ethyl mercaptan, is an organosulfur compound with the formula CH3CH2SH. It is a colorless liquid with a distinct odor. Abbreviated EtSH, it consists of an ethyl group (Et), CH3CH2, attached to a thiol group, SH. Its structure parallels that of ethanol, but with sulfur in place of oxygen. The odor of EtSH is infamous. Ethanethiol is more volatile than ethanol due to a diminished ability to engage in hydrogen bonding. Ethanethiol is toxic in high concentrations. It occurs naturally as a minor component of petroleum, and may be added to otherwise odorless gaseous products such as liquefied petroleum gas (LPG) to help warn of gas leaks. At these concentrations, ethanethiol is not harmful.

An aroma compound, also known as an odorant, aroma, fragrance or flavoring, is a chemical compound that has a smell or odor. For an individual chemical or class of chemical compounds to impart a smell or fragrance, it must be sufficiently volatile for transmission via the air to the olfactory system in the upper part of the nose. As examples, various fragrant fruits have diverse aroma compounds, particularly strawberries which are commercially cultivated to have appealing aromas, and contain several hundred aroma compounds.

Petroleum products are materials derived from crude oil (petroleum) as it is processed in oil refineries. Unlike petrochemicals, which are a collection of well-defined usually pure organic compounds, petroleum products are complex mixtures. Most petroleum is converted into petroleum products, which include several classes of fuels.

A stink bomb, sometimes called a stinkpot, is a device designed to create an unpleasant smell. They range in effectiveness from being used as simple pranks to military grade malodorants or riot control chemical agents.

Thiophenol is an organosulfur compound with the formula C6H5SH, sometimes abbreviated as PhSH. This foul-smelling colorless liquid is the simplest aromatic thiol. The chemical structures of thiophenol and its derivatives are analogous to phenols, where the oxygen atom in the hydroxyl group (-OH) bonded to the aromatic ring in phenol is replaced by a sulfur atom. The prefix thio- implies a sulfur-containing compound and when used before a root word name for a compound which would normally contain an oxygen atom, in the case of 'thiol' that the alcohol oxygen atom is replaced by a sulfur atom.
Extreme pressure additives, or EP additives, are additives for lubricants with a role to decrease wear of the parts of the gears exposed to very high pressures. They are also added to cutting fluids for machining of metals.
Grapefruit mercaptan is a natural organic compound found in grapefruit. It is a monoterpenoid that contains a thiol functional group. Structurally a hydroxy group of terpineol is replaced by the thiol in grapefruit mercaptan, so it also called thioterpineol. Volatile thiols typically have very strong, often unpleasant odors that can be detected by humans in very low concentrations. Grapefruit mercaptan has a very potent, but not unpleasant, odor, and it is the chemical constituent primarily responsible for the aroma of grapefruit. This characteristic aroma is a property of only the R enantiomer.

Butane-1-thiol, also known as butyl mercaptan, is a volatile, clear to yellowish liquid with a fetid odor, commonly described as "skunk" odor. In fact, 1-butanethiol is structurally similar to several major constituents of a skunk's defensive spray but is not actually present in the spray. The scent of 1-butanethiol is so strong that the human nose can easily detect it in the air at concentrations as low as 10 parts per billion. The threshold level for 1-butanethiol is reported as 1.4 ppb
Oil additives are chemical compounds that improve the lubricant performance of base oil. The manufacturer of many oils can use the same base stock for each formulation and can choose different additives for each use. Additives comprise up to 5% by weight of some oils.
The Lubrizol Corporation is an American provider of specialty chemicals for the transportation, industrial and consumer markets. These products include additives for engine oils and other transportation-related fluids, additives for industrial lubricants, and additives for gasoline and diesel fuel. In addition, Lubrizol makes ingredients and additives for personal care products, pharmaceuticals and medical devices, specialty materials, including plastics technology, and coatings in the form of specialty resins and additives.

Automotive oil recycling involves the recycling of used oils and the creation of new products from the recycled oils, and includes the recycling of motor oil and hydraulic oil. Oil recycling also benefits the environment: increased opportunities for consumers to recycle oil lessens the likelihood of used oil being dumped on lands and in waterways. For example, one gallon of motor oil dumped into waterways has the potential to pollute one million gallons of water.

4-Methyl-2-pentanol or methyl isobutyl carbinol (MIBC) is an organic chemical compound used primarily as a frother in mineral flotation and in the production of lubricant oil additives such as Zinc dithiophosphate. It is also used as a solvent, in organic synthesis, and in the manufacture of brake fluid and as a precursor to some plasticizers. It is an acetone derivative in liquid state, with limited solubility in water but generally miscible with most organic solvents.

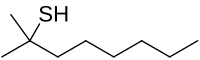
tert-Butylthiol, also known as tert-butyl mercaptan (TBM), and abbreciated t-BuSH, is an organosulfur compound with the formula (CH3)3CSH. This thiol has a strong odor. It is considered a flavoring agent.
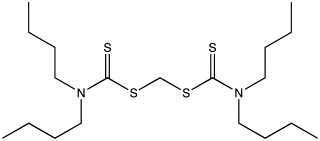
In chemistry, a thioxanthate is an organosulfur compound with the formula RSCS2X. When X is an alkali metal, the thioxanthate is a salt. When X is a transition metal, the thioxanthate is a ligand, and when X is an organic group, the compounds are called thioxanthate esters. They are usually yellow colored compounds that often dissolve in organic solvents. They are used as precursors to some catalysts, froth flotation agents, and additives for lubricants.