
Amyl nitrite is a chemical compound with the formula C5H11ONO. A variety of isomers are known, but they all feature an amyl group attached to the nitrite functional group. The alkyl group is unreactive and the chemical and biological properties are mainly due to the nitrite group. Like other alkyl nitrites, amyl nitrite is bioactive in mammals, being a vasodilator, which is the basis of its use as a prescription medicine. As an inhalant, it also has a psychoactive effect, which has led to its recreational use, with its smell being described as that of old socks or dirty feet. It is also referred to as banapple gas.

Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion does not always result in fire, because a flame is only visible when substances undergoing combustion vaporize, but when it does, a flame is a characteristic indicator of the reaction. While activation energy must be supplied to initiate combustion, the heat from a flame may provide enough energy to make the reaction self-sustaining.
Reaction kinetics in uniform supersonic flow is an experiment investigating chemical reactions taking place at very low temperatures.
A chain reaction is a sequence of reactions where a reactive product or by-product causes additional reactions to take place. In a chain reaction, positive feedback leads to a self-amplifying chain of events.
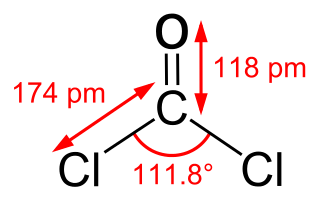
Phosgene is an organic chemical compound with the formula COCl2. It is a toxic, colorless gas; in low concentrations, its musty odor resembles that of freshly cut hay or grass. It can be thought of chemically as the double acyl chloride analog of carbonic acid, or structurally as formaldehyde with the hydrogen atoms replaced by chlorine atoms. Phosgene is a valued and important industrial building block, especially for the production of precursors of polyurethanes and polycarbonate plastics.
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is the first chemical step in photosynthesis, is called carboxylation, the addition of CO2 to a compound. Enzymes that catalyze decarboxylations are called decarboxylases or, the more formal term, carboxy-lyases (EC number 4.1.1).

Peroxyacetyl nitrate is a peroxyacyl nitrate. It is a secondary pollutant present in photochemical smog. It is thermally unstable and decomposes into peroxyethanoyl radicals and nitrogen dioxide gas. It is a lachrymatory substance, meaning that it irritates the lungs and eyes.

The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per unit time. Reaction rates can vary dramatically. For example, the oxidative rusting of iron under Earth's atmosphere is a slow reaction that can take many years, but the combustion of cellulose in a fire is a reaction that takes place in fractions of a second. For most reactions, the rate decreases as the reaction proceeds. A reaction's rate can be determined by measuring the changes in concentration over time.
Chemical kinetics, also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is different from chemical thermodynamics, which deals with the direction in which a reaction occurs but in itself tells nothing about its rate. Chemical kinetics includes investigations of how experimental conditions influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition states, as well as the construction of mathematical models that also can describe the characteristics of a chemical reaction.

Dinitrogen pentoxide is the chemical compound with the formula N2O5. It is one of the binary nitrogen oxides, a family of compounds that only contain nitrogen and oxygen. It exists as colourless crystals that sublime slightly above room temperature, yielding a colorless gas.
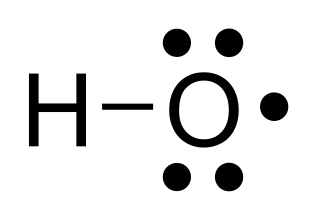
The hydroxyl radical is the diatomic molecule •
OH. The hydroxyl radical is very stable as a dilute gas, but it decays very rapidly in the condensed phase. It is pervasive in some situations. Most notably the hydroxyl radicals are produced from the decomposition of hydroperoxides (ROOH) or, in atmospheric chemistry, by the reaction of excited atomic oxygen with water. It is also important in the field of radiation chemistry, since it leads to the formation of hydrogen peroxide and oxygen, which can enhance corrosion and SCC in coolant systems subjected to radioactive environments. The unpaired electron of the hydroxyl radical is officially represented by a middle dot, •, beside the O.

Heterogeneous catalysis is catalysis where the phase of catalysts differs from that of the reactants or products. The process contrasts with homogeneous catalysis where the reactants, products and catalyst exist in the same phase. Phase distinguishes between not only solid, liquid, and gas components, but also immiscible mixtures, or anywhere an interface is present.
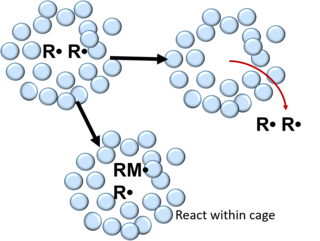
In chemistry, the cage effect (also known as geminate recombination) describes how the properties of a molecule are affected by its surroundings. First introduced by James Franck and Eugene Rabinowitch in 1934, the cage effect suggests that instead of acting as an individual particle, molecules in solvent are more accurately described as an encapsulated particle. The encapsulated molecules or radicals are called cage pairs or geminate pairs. In order to interact with other molecules, the caged particle must diffuse from its solvent cage. The typical lifetime of a solvent cage is 10-11 seconds. Many manifestations of the cage effect exist.

Ethylbenzene is an organic compound with the formula C6H5CH2CH3. It is a highly flammable, colorless liquid with an odor similar to that of gasoline. This monocyclic aromatic hydrocarbon is important in the petrochemical industry as a reaction intermediate in the production of styrene, the precursor to polystyrene, a common plastic material. In 2012, more than 99% of ethylbenzene produced was consumed in the production of styrene.
A free-radical reaction is any chemical reaction involving free radicals. This reaction type is abundant in organic reactions. Two pioneering studies into free radical reactions have been the discovery of the triphenylmethyl radical by Moses Gomberg (1900) and the lead-mirror experiment described by Friedrich Paneth in 1927. In this last experiment tetramethyllead is decomposed at elevated temperatures to methyl radicals and elemental lead in a quartz tube. The gaseous methyl radicals are moved to another part of the chamber in a carrier gas where they react with lead in a mirror film which slowly disappears.
Gas phase ion chemistry is a field of science encompassed within both chemistry and physics. It is the science that studies ions and molecules in the gas phase, most often enabled by some form of mass spectrometry. By far the most important applications for this science is in studying the thermodynamics and kinetics of reactions. For example, one application is in studying the thermodynamics of the solvation of ions. Ions with small solvation spheres of 1, 2, 3... solvent molecules can be studied in the gas phase and then extrapolated to bulk solution.
The Haber–Weiss reaction generates •OH (hydroxyl radicals) from H2O2 (hydrogen peroxide) and superoxide (•O2−) catalyzed by iron ions. It was first proposed by Fritz Haber and his student Joseph Joshua Weiss in 1932.
In chemical kinetics, the Lindemann mechanism is a schematic reaction mechanism for unimolecular reactions. Frederick Lindemann and J. A. Christiansen proposed the concept almost simultaneously in 1921, and Cyril Hinshelwood developed it to take into account the energy distributed among vibrational degrees of freedom for some reaction steps.
Keith James Laidler, born in England, was notable as a pioneer in chemical kinetics and authority on the physical chemistry of enzymes.
1,2-Difluoroethane is a saturated hydrofluorocarbon containing an atom of fluorine attached to each of two carbons atoms. The formula can be written CH2FCH2F. It is an isomer of 1,1-difluoroethane. It has a HFC name of HFC-152 with no letter suffix. When cooled to cryogenic temperatures it can have different conformers, gauche and trans. In the liquid form these are about equally abundant and easily interconvert. As a gas it is mostly the gauche form.










