A dopamine reuptake inhibitor (DRI) is a class of drug which acts as a reuptake inhibitor of the monoamine neurotransmitter dopamine by blocking the action of the dopamine transporter (DAT). Reuptake inhibition is achieved when extracellular dopamine not absorbed by the postsynaptic neuron is blocked from re-entering the presynaptic neuron. This results in increased extracellular concentrations of dopamine and increase in dopaminergic neurotransmission.

Tropinone is an alkaloid, famously synthesised in 1917 by Robert Robinson as a synthetic precursor to atropine, a scarce commodity during World War I. Tropinone and the alkaloids cocaine and atropine all share the same tropane core structure. Its corresponding conjugate acid at pH 7.3 major species is known as tropiniumone.

WIN 35,428 is a stimulant drug used in scientific research. CFT is a phenyltropane based dopamine reuptake inhibitor and is structurally derived from cocaine. It is around 3-10x more potent than cocaine and lasts around 7 times longer based on animal studies. While the naphthalenedisulfonate salt is the most commonly used form in scientific research due to its high solubility in water, the free base and hydrochloride salts are known compounds and can also be produced. The tartrate is another salt form that is reported.

Vanoxerine is an investigational drug which is being evaluated for the treatment of heart arrhythmias and cocaine dependence. Vanoxerine is a piperazine derivative which has multiple pharmacological activities including acting as an dopamine reuptake inhibitor, serotonin transporter inhibitor, and as a blocker of the cardiac hERG repolarizing potassium channel (IKr).
Phenyltropanes (PTs) were originally developed to reduce cocaine addiction and dependency. In general these compounds act as inhibitors of the plasmalemmal monoamine reuptake transporters. This research has spanned beyond the last couple decades, and has picked up its pace in recent times, creating numerous phenyltropanes as research into cocaine analogues garners interest to treat addiction.

(+)-CPCA is a stimulant drug similar in structure to pethidine and to RTI-31, but nocaine lacks the two-carbon bridge of RTI-31's tropane skeleton. This compound was first developed as a substitute agent for cocaine.

Troparil is a stimulant drug used in scientific research. Troparil is a phenyltropane-based dopamine reuptake inhibitor (DRI) that is derived from methylecgonidine. Troparil is a few times more potent than cocaine as a dopamine reuptake inhibitor, but is less potent as a serotonin reuptake inhibitor, and has a duration spanning a few times longer, since the phenyl ring is directly connected to the tropane ring through a non-hydrolyzable carbon-carbon bond. The lack of an ester linkage removes the local anesthetic action from the drug, so troparil is a pure stimulant. This change in activity also makes troparil slightly less cardiotoxic than cocaine. The most commonly used form of troparil is the tartrate salt, but the hydrochloride and naphthalenedisulfonate salts are also available, as well as the free base.
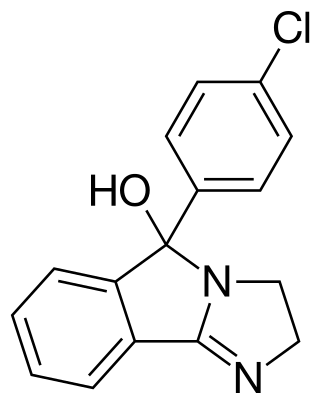
Mazindol, sold under the brand names Mazanor and Sanorex, is a central nervous system (CNS) stimulant which is used as an appetite suppressant. It was developed by Sandoz-Wander in the 1960s. The US Food and Drug Administration approved mazindol in June 1973, but Novartis, the manufacturer, discontinued it in 1999 for reasons unrelated to its efficacy or safety.

2-Benzylpiperidine is a stimulant drug of the arylpiperidine family. It is similar in structure to certain other stimulants such as methylphenidate and desoxypipradrol. However, it is far less potent as a monoamine reuptake inhibitor in comparison. The drug is little used as a stimulant, with its main use being as a synthetic intermediate in the manufacture of other drugs.

RTI-126 is a phenyltropane derivative which acts as a potent monoamine reuptake inhibitor and stimulant drug, and has been sold as a designer drug. It is around 5 times more potent than cocaine at inhibiting monoamine reuptake in vitro, but is relatively unselective. It binds to all three monoamine transporters, although still with some selectivity for the dopamine transporter. RTI-126 has a fast onset of effects and short duration of action, and its pharmacological profile in animals is among the closest to cocaine itself out of all the drugs in the RTI series. Its main application in scientific research has been in studies investigating the influence of pharmacokinetics on the abuse potential of stimulant drugs, with its rapid entry into the brain thought to be a key factor in producing its high propensity for development of dependence in animals.
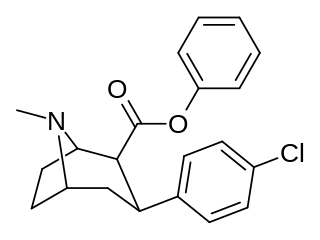
RTI(-4229)-113 is a stimulant drug which acts as a potent and fully selective dopamine reuptake inhibitor (DRI). It has been suggested as a possible substitute drug for the treatment of cocaine addiction. "RTI-113 has properties that make it an ideal medication for cocaine abusers, such as an equivalent efficacy, a higher potency, and a longer duration of action as compared to cocaine." Replacing the methyl ester in RTI-31 with a phenyl ester makes the resultant RTI-113 fully DAT specific. RTI-113 is a particularly relevant phenyltropane cocaine analog that has been tested on squirrel monkeys. RTI-113 has also been tested against cocaine in self-administration studies for DAT occupancy by PET on awake rhesus monkeys. The efficacy of cocaine analogs to elicit self-administration is closely related to the rate at which they are administered. Slower onset of action analogs are less likely to function as positive reinforcers than analogues that have a faster rate of onset.

RTI(-4229)-177 is a synthetic stimulant drug from the phenyltropane family, which acts as a DRI with micromolar affinity for the SERT. RTI-177 has an unusually long duration of action of 20 hours or more, substantially longer than the related compound RTI-336 from which it differs in molecular structure only by the absence of a p-methyl group.

RTI-31 is a synthetic analog of cocaine that acts as a stimulant. Semi-synthesis of this compound is dependent upon the availability of cocaine starting material. According to the article, RTI-31 is 64 times the strength of cocaine in terms of its potency to elicit self-administration in monkeys. WIN 35428 was 6 times weaker than RTI-31, whereas RTI-51 was 2.6 times weaker than RTI-31.

(–)-2β-Carbomethoxy-3β-(4-bromophenyl)tropane is a semi-synthetic alkaloid in the phenyltropane group of psychostimulant compounds. First publicized in the 1990s, it has not been used enough to have gained a fully established profile. RTI-51 can be expected to have properties lying somewhere in between RTI-31 and RTI-55. It has a ratio of monoamine reuptake inhibition of dopamine > serotonin > norepinephrine which is an unusual balance of effects not produced by other commonly used compounds. It has been used in its 76Br radiolabelled form to map the distribution of dopamine transporters in the brain.
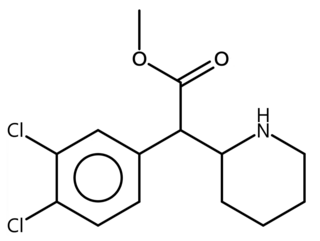
3,4-dichloromethylphenidate is a potent stimulant drug from the phenidate class closely related to methylphenidate. It acts as a potent serotonin-norepinephrine-dopamine reuptake inhibitor with a long duration of action. It has been sold online as a designer drug.
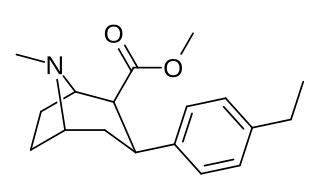
RTI-83 is a phenyltropane derivative which represents a rare example of an SDRI or serotonin-dopamine reuptake inhibitor, a drug which inhibits the reuptake of the neurotransmitters serotonin and dopamine, while having little or no effect on the reuptake of the related neurotransmitter noradrenaline. With a binding affinity (Ki) of 55 nM at DAT and 28.4 nM at SERT but only 4030 nM at NET, RTI-83 has reasonable selectivity for DAT/SERT over NET

4-Fluoromethylphenidate is a stimulant drug that acts as a higher potency dopamine reuptake inhibitor than the closely related methylphenidate.

Flazalone is an anti-inflammatory drug that has not been approved as a medicine.

















