Adrenocortical dysplasia protein homolog is a protein that in humans is encoded by the ACD gene. [5] [6] [7]
Adrenocortical dysplasia protein homolog is a protein that in humans is encoded by the ACD gene. [5] [6] [7]
This gene encodes a protein that is involved in telomere function. This protein is one of six core proteins in the telosome/shelterin telomeric complex, which functions to maintain telomere length and to protect telomere ends. Through its interaction with other components, this protein plays a key role in the assembly and stabilization of this complex, and it mediates the access of telomerase to the telomere. Multiple transcript variants encoding different isoforms have been found for this gene. This gene, which is also referred to as TPP1, is distinct from the unrelated TPP1 gene on chromosome 11, which encodes tripeptidyl-peptidase I. [7]
TPP1 is a component of the telomere-specific shelterin complex, which facilitates the replication of the double-stranded telomeric DNA tracts and protects the telomeric end from unregulated DNA repair activities. TPP1 mainly functions as a regulator of telomerase recruitment, activation, and regulation. [8] Although TPP1 was originally described as a bridging factor between TRF1 and TRF2, which participate in a pathway with POT1 as a negative regulator of telomerase-dependent telomere length control, [9] more recent studies suggest that TPP1 could directly promotes telomerase activity at the telomere. [10] TPP1 is both necessary and sufficient to recruit the telomerase enzyme to telomeres, and is the only shelterin protein in direct contact with telomerase. [11]
A part of the TPP1 oligonucleotide/oligosaccharide-binding (OB) fold named TEL patch that interacts with the catalytic subunit of telomerase, hTERT, has been proven essential for telomerase activation. [12] TPP1 has also been demonstrated as the only pathway required for recruitment of telomerase to chromosome ends, and it also defines telomere length homeostasis in hESCs. [12]
ACD (gene) has been shown to interact with POT1 [5] [6] [13] and TINF2. [5] [6]
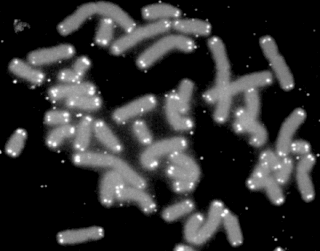
A telomere is a region of repetitive nucleotide sequences associated with specialized proteins at the ends of linear chromosomes. Telomeres are a widespread genetic feature most commonly found in eukaryotes. In most, if not all species possessing them, they protect the terminal regions of chromosomal DNA from progressive degradation and ensure the integrity of linear chromosomes by preventing DNA repair systems from mistaking the very ends of the DNA strand for a double-strand break.

Telomerase reverse transcriptase is a catalytic subunit of the enzyme telomerase, which, together with the telomerase RNA component (TERC), comprises the most important unit of the telomerase complex.
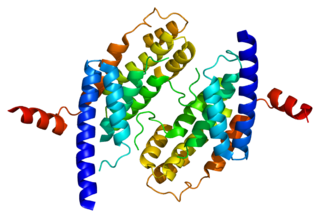
Telomeric repeat-binding factor 2 is a protein that is present at telomeres throughout the cell cycle. It is also known as TERF2, TRF2, and TRBF2, and is encoded in humans by the TERF2 gene. It is a component of the shelterin nucleoprotein complex and a second negative regulator of telomere length, playing a key role in the protective activity of telomeres. It was first reported in 1997 in the lab of Titia de Lange, where a DNA sequence similar, but not identical, to TERF1 was discovered, with respect to the Myb-domain. De Lange isolated the new Myb-containing protein sequence and called it TERF2.

Telomeric repeat-binding factor 1 is a protein that in humans is encoded by the TERF1 gene.

Telomerase RNA component, also known as TR, TER or TERC, is an ncRNA found in eukaryotes that is a component of telomerase, the enzyme used to extend telomeres. TERC serves as a template for telomere replication by telomerase. Telomerase RNAs differ greatly in sequence and structure between vertebrates, ciliates and yeasts, but they share a 5' pseudoknot structure close to the template sequence. The vertebrate telomerase RNAs have a 3' H/ACA snoRNA-like domain.

Protection of telomeres protein 1 is a protein that in humans is encoded by the POT1 gene.
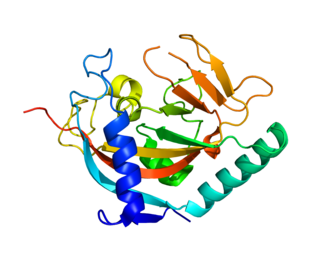
Tankyrase, also known as tankyrase 1, is an enzyme that in humans is encoded by the TNKS gene. It inhibits the binding of TERF1 to telomeric DNA. Tankyrase attracts substantial interest in cancer research through its interaction with AXIN1 and AXIN2, which are negative regulators of pro-oncogenic β-catenin signaling. Importantly, activity in the β-catenin destruction complex can be increased by tankyrase inhibitors and thus such inhibitors are a potential therapeutic option to reduce the growth of β-catenin-dependent cancers.

TERF1-interacting nuclear factor 2 is a protein that in humans is encoded by the TINF2 gene. TINF2 is a component of the shelterin protein complex found at the end of telomeres.

Telomeric repeat-binding factor 2-interacting protein 1 also known as repressor activator protein 1 (Rap1) is a protein that in humans is encoded by the TERF2IP gene.
PIN2/TERF1-interacting telomerase inhibitor 1, also known as PINX1, is a human gene. PINX1 is also known as PIN2 interacting protein 1. PINX1 is a telomerase inhibitor and a possible tumor suppressor.
Telomere-binding proteins function to bind telomeric DNA in various species. In particular, telomere-binding protein refers to TTAGGG repeat binding factor-1 (TERF1) and TTAGGG repeat binding factor-2 (TERF2). Telomere sequences in humans are composed of TTAGGG sequences which provide protection and replication of chromosome ends to prevent degradation. Telomere-binding proteins can generate a T-loop to protect chromosome ends. TRFs are double-stranded proteins which are known to induce bending, looping, and pairing of DNA which aids in the formation of T-loops. They directly bind to TTAGGG repeat sequence in the DNA. There are also subtelomeric regions present for regulation. However, in humans, there are six subunits forming a complex known as shelterin.
Shelterin is a protein complex known to protect telomeres in many eukaryotes from DNA repair mechanisms, as well as to regulate telomerase activity. In mammals and other vertebrates, telomeric DNA consists of repeating double-stranded 5'-TTAGGG-3' (G-strand) sequences along with the 3'-AATCCC-5' (C-strand) complement, ending with a 50-400 nucleotide 3' (G-strand) overhang. Much of the final double-stranded portion of the telomere forms a T-loop (Telomere-loop) that is invaded by the 3' (G-strand) overhang to form a small D-loop (Displacement-loop).

Homeobox containing 1, also known as homeobox telomere-binding protein 1 (HOT1), is a protein that in humans is encoded by the HMBOX1 gene. HMBOX1 directly binds to the double-stranded repeat sequence of telomeres.

Titia de Lange is the Director of the Anderson Center for Cancer Research, the Leon Hess professor and the head of Laboratory Cell Biology and Genetics at Rockefeller University.

Telomeric repeat–containing RNA (TERRA) is a long non-coding RNA transcribed from telomeres - repetitive nucleotide regions found on the ends of chromosomes that function to protect DNA from deterioration or fusion with neighboring chromosomes. TERRA has been shown to be ubiquitously expressed in almost all cell types containing linear chromosomes - including humans, mice, and yeasts. While the exact function of TERRA is still an active area of research, it is generally believed to play a role in regulating telomerase activity as well as maintaining the heterochromatic state at the ends of chromosomes. TERRA interaction with other associated telomeric proteins has also been shown to help regulate telomere integrity in a length-dependent manner.

María Antonia Blasco Marhuenda, known as María Blasco, is a Spanish molecular biologist. She is the current director of the Spanish National Cancer Research Centre.
TERRA in biology is an abbreviation for "TElomeric Repeat-containing RNA". TERRA is RNA that is transcribed from telomeres — the repeating 6-nucleotide sequences that cap the ends of chromosomes. TERRA functions with shelterin to inhibit telomere lengthening by enzyme telomerase.
The CST complex is a cellular multiprotein complex involved in telomere maintenance. In budding yeast, it is composed of the proteins Cdc13, Stn1, and Ten1; in mammals, it consists of the proteins CTC1, STN1, and TEN1. It is related to the replication protein A complex.
Telomeres, the caps on the ends of eukaryotic chromosomes, play critical roles in cellular aging and cancer. An important facet to how telomeres function in these roles is their involvement in cell cycle regulation.

Joachim Lingner is a Swiss molecular biologist. He holds the professorship for life sciences and leads the Lingner Lab at the École Polytechnique Fédérale de Lausanne (EPFL).