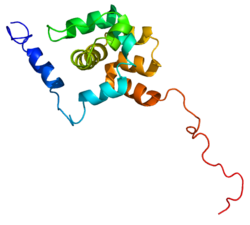
Proteasomes are protein complexes which degrade unneeded or damaged proteins by proteolysis, a chemical reaction that breaks peptide bonds. Enzymes that help such reactions are called proteases.

26S protease regulatory subunit 6A, also known as 26S proteasome AAA-ATPase subunit Rpt5, is an enzyme that in humans is encoded by the PSMC3 gene. This protein is one of the 19 essential subunits of a complete assembled 19S proteasome complex Six 26S proteasome AAA-ATPase subunits together with four non-ATPase subunits form the base sub complex of 19S regulatory particle for proteasome complex.

Proteasome subunit alpha type-4 also known as macropain subunit C9, proteasome component C9, and 20S proteasome subunit alpha-3 is a protein that in humans is encoded by the PSMA4 gene. This protein is one of the 17 essential subunits that contributes to the complete assembly of 20S proteasome complex.

26S proteasome non-ATPase regulatory subunit 13 is an enzyme that in humans is encoded by the PSMD13 gene.

26S proteasome non-ATPase regulatory subunit 12 is an enzyme that in humans is encoded by the PSMD12 gene.

Proteasome subunit beta type-3, also known as 20S proteasome subunit beta-3, is a protein that in humans is encoded by the PSMB3 gene. This protein is one of the 17 essential subunits that contribute to the complete assembly of the 20S proteasome complex. In particular, proteasome subunit beta type-2, along with other beta subunits, assemble into two heptameric rings and subsequently a proteolytic chamber for substrate degradation. The eukaryotic proteasome recognizes degradable proteins, including damaged proteins for protein quality control purpose or key regulatory protein components for dynamic biological processes.
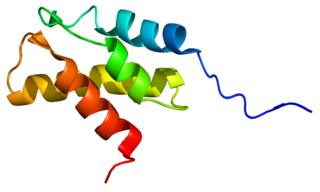
26S protease regulatory subunit 8, also known as 26S proteasome AAA-ATPase subunit Rpt6, is an enzyme that in humans is encoded by the PSMC5 gene. This protein is one of the 19 essential subunits of a complete assembled 19S proteasome complex Six 26S proteasome AAA-ATPase subunits together with four non-ATPase subunits form the base sub complex of 19S regulatory particle for proteasome complex.

26S proteasome non-ATPase regulatory subunit 4, also as known as 26S Proteasome Regulatory Subunit Rpn10, is an enzyme that in humans is encoded by the PSMD4 gene. This protein is one of the 19 essential subunits that contributes to the complete assembly of 19S proteasome complex.

26S protease regulatory subunit 7, also known as 26S proteasome AAA-ATPase subunit Rpt1, is an enzyme that in humans is encoded by the PSMC2 gene This protein is one of the 19 essential subunits of a complete assembled 19S proteasome complex. Six 26S proteasome AAA-ATPase subunits together with four non-ATPase subunits form the base sub complex of 19S regulatory particle for proteasome complex.

Proteasome subunit alpha type-1 is a protein that in humans is encoded by the PSMA1 gene. This protein is one of the 17 essential subunits that contributes to the complete assembly of 20S proteasome complex.
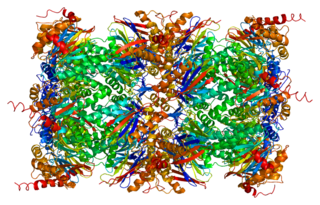
Proteasome subunit beta type-5 also known as 20S proteasome subunit beta-5 is a protein that in humans is encoded by the PSMB5 gene. This protein is one of the 17 essential subunits that contributes to the complete assembly of 20S proteasome complex. In particular, proteasome subunit beta type-5, along with other beta subunits, assemble into two heptameric rings and subsequently a proteolytic chamber for substrate degradation. This protein contains "chymotrypsin-like" activity and is capable of cleaving after large hydrophobic residues of peptide. The eukaryotic proteasome recognized degradable proteins, including damaged proteins for protein quality control purpose or key regulatory protein components for dynamic biological processes. An essential function of a modified proteasome, the immunoproteasome, is the processing of class I MHC peptides.

26S protease regulatory subunit 4, also known as 26S proteasome AAA-ATPase subunit Rpt2, is an enzyme that in humans is encoded by the PSMC1 gene. This protein is one of the 19 essential subunits of a complete assembled 19S proteasome complex. Six 26S proteasome AAA-ATPase subunits together with four non-ATPase subunits form the base sub complex of 19S regulatory particle for proteasome complex.
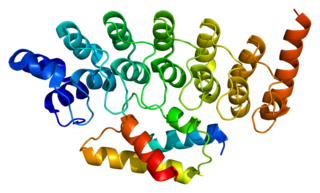
26S protease regulatory subunit 6B, also known as 26S proteasome AAA-ATPase subunit Rpt3, is an enzyme that in humans is encoded by the PSMC4 gene. This protein is one of the 19 essential subunits of a complete assembled 19S proteasome complex Six 26S proteasome AAA-ATPase subunits together with four non-ATPase subunits form the base sub complex of 19S regulatory particle for proteasome complex.

26S proteasome non-ATPase regulatory subunit 7, also known as 26S proteasome non-ATPase subunit Rpn8, is an enzyme that in humans is encoded by the PSMD7 gene.

26S protease regulatory subunit S10B, also known as 26S proteasome AAA-ATPase subunit Rpt4, is an enzyme that in humans is encoded by the PSMC6 gene. This protein is one of the 19 essential subunits of a complete assembled 19S proteasome complex Six 26S proteasome AAA-ATPase subunits together with four non-ATPase subunits form the base sub complex of 19S regulatory particle for proteasome complex.

26S proteasome non-ATPase regulatory subunit 1, also as known as 26S Proteasome Regulatory Subunit Rpn2, is a protein that in humans is encoded by the PSMD1 gene. This protein is one of the 19 essential subunits that contributes to the complete assembly of 19S proteasome complex.

26S proteasome non-ATPase regulatory subunit 2, also as known as 26S Proteasome Regulatory Subunit Rpn1, is an enzyme that in humans is encoded by the PSMD2 gene.

26S proteasome non-ATPase regulatory subunit 11 is an enzyme that in humans is encoded by the PSMD11 gene.

26S proteasome non-ATPase regulatory subunit 8 is an enzyme that in humans is encoded by the PSMD8 gene.

26S proteasome non-ATPase regulatory subunit 14, also known as 26S proteasome non-ATPase subunit Rpn11, is an enzyme that in humans is encoded by the PSMD14 gene. This protein is one of the 19 essential subunits of the complete assembled 19S proteasome complex. Nine subunits Rpn3, Rpn5, Rpn6, Rpn7, Rpn8, Rpn9, Rpn11, SEM1, and Rpn12 form the lid sub complex of the 19S regulatory particle of the proteasome complex.