
The cytoskeleton is a complex, dynamic network of interlinking protein filaments present in the cytoplasm of all cells, including those of bacteria and archaea. In eukaryotes, it extends from the cell nucleus to the cell membrane and is composed of similar proteins in the various organisms. It is composed of three main components: microfilaments, intermediate filaments, and microtubules, and these are all capable of rapid growth or disassembly depending on the cell's requirements.

Cytokinesis is the part of the cell division process and part of mitosis during which the cytoplasm of a single eukaryotic cell divides into two daughter cells. Cytoplasmic division begins during or after the late stages of nuclear division in mitosis and meiosis. During cytokinesis the spindle apparatus partitions and transports duplicated chromatids into the cytoplasm of the separating daughter cells. It thereby ensures that chromosome number and complement are maintained from one generation to the next and that, except in special cases, the daughter cells will be functional copies of the parent cell. After the completion of the telophase and cytokinesis, each daughter cell enters the interphase of the cell cycle.

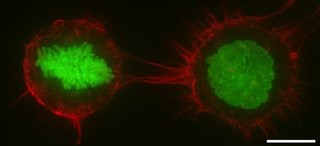
In cell biology, the cleavage furrow is the indentation of the cell's surface that begins the progression of cleavage, by which animal and some algal cells undergo cytokinesis, the final splitting of the membrane, in the process of cell division. The same proteins responsible for muscle contraction, actin and myosin, begin the process of forming the cleavage furrow, creating an actomyosin ring. Other cytoskeletal proteins and actin binding proteins are involved in the procedure.

Motor proteins are a class of molecular motors that can move along the cytoplasm of cells. They convert chemical energy into mechanical work by the hydrolysis of ATP. Flagellar rotation, however, is powered by a proton pump.
The cell cortex, also known as the actin cortex, cortical cytoskeleton or actomyosin cortex, is a specialized layer of cytoplasmic proteins on the inner face of the cell membrane. It functions as a modulator of membrane behavior and cell surface properties. In most eukaryotic cells lacking a cell wall, the cortex is an actin-rich network consisting of F-actin filaments, myosin motors, and actin-binding proteins. The actomyosin cortex is attached to the cell membrane via membrane-anchoring proteins called ERM proteins that plays a central role in cell shape control. The protein constituents of the cortex undergo rapid turnover, making the cortex both mechanically rigid and highly plastic, two properties essential to its function. In most cases, the cortex is in the range of 100 to 1000 nanometers thick.
An asymmetric cell division produces two daughter cells with different cellular fates. This is in contrast to symmetric cell divisions which give rise to daughter cells of equivalent fates. Notably, stem cells divide asymmetrically to give rise to two distinct daughter cells: one copy of the original stem cell as well as a second daughter programmed to differentiate into a non-stem cell fate.
The LIM kinases are a family of actin-binding kinases that phosphorylate members of the ADF/cofilin family of actin binding and filament severing proteins. The LIM kinase family is made up of two proteins: LIM kinase-1 (LIMK1) and LIM kinase-2 (LIMK2)
Septins are a group of GTP-binding proteins expressed in all eukaryotic cells except plants. Different septins form protein complexes with each other. These complexes can further assemble into filaments, rings and gauzes. Assembled as such, septins function in cells by localizing other proteins, either by providing a scaffold to which proteins can attach, or by forming a barrier preventing the diffusion of molecules from one compartment of the cell to another, or in the cell cortex as a barrier to the diffusion of membrane-bound proteins.

Aurora kinase B is a protein that functions in the attachment of the mitotic spindle to the centromere.
Polo-like kinases (Plks) are regulatory serine/threonine kinases of the cell cycle involved in mitotic entry, mitotic exit, spindle formation, cytokinesis, and meiosis. Only one Plk is found in the genomes of the fly Drosophila melanogaster (Polo), budding yeast (Cdc5) and fission yeast (Plo1). Vertebrates and other animals, however, have many Plk family members including Plk1, Plk2/Snk, Plk3/Prk/FnK, Plk4/Sak and Plk5. Of the vertebrate Plk family members, the mammalian Plk1 has been most extensively studied. During mitosis and cytokinesis, Plks associate with several structures including the centrosome, kinetochores, and the central spindle.

Transforming protein RhoA, also known as Ras homolog family member A (RhoA), is a small GTPase protein in the Rho family of GTPases that in humans is encoded by the RHOA gene. While the effects of RhoA activity are not all well known, it is primarily associated with cytoskeleton regulation, mostly actin stress fibers formation and actomyosin contractility. It acts upon several effectors. Among them, ROCK1 and DIAPH1 are the best described. RhoA, and the other Rho GTPases, are part of a larger family of related proteins known as the Ras superfamily, a family of proteins involved in the regulation and timing of cell division. RhoA is one of the oldest Rho GTPases, with homologues present in the genomes since 1.5 billion years. As a consequence, RhoA is somehow involved in many cellular processes which emerged throughout evolution. RhoA specifically is regarded as a prominent regulatory factor in other functions such as the regulation of cytoskeletal dynamics, transcription, cell cycle progression and cell transformation.

Myosin-10 also known as myosin heavy chain 10 or non-muscle myosin IIB (NM-IIB) is a protein that in humans is encoded by the MYH10 gene. Non-muscle myosins are expressed in a wide variety of tissues, but NM-IIB is the only non-muscle myosin II isoform expressed in cardiac muscle, where it localizes to adherens junctions within intercalated discs. NM-IIB is essential for normal development of cardiac muscle and for integrity of intercalated discs. Mutations in MYH10 have been identified in patients with left atrial enlargement.

Citron Rho-interacting kinase is an enzyme that in humans is encoded by the CIT gene.

Septin-7 is a protein that in humans is encoded by the SEPT7 gene.
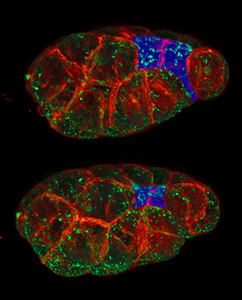
Apical constriction is the process in which contraction of the apical side of a cell causes the cell to take on a wedged shape. Generally, this shape change is coordinated across many cells of an epithelial layer, generating forces that can bend or fold the cell sheet.

Rho-associated protein kinase (ROCK) is a kinase belonging to the AGC family of serine-threonine specific protein kinases. It is involved mainly in regulating the shape and movement of cells by acting on the cytoskeleton.
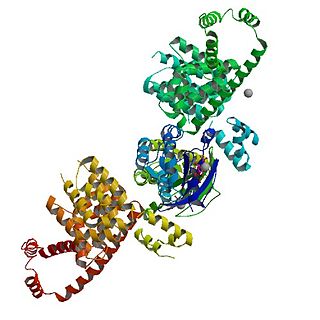
mDia1 is a member of the protein family called the formins and is a Rho effector. It is the mouse version of the diaphanous homolog 1 of Drosophila. mDia1 localizes to cells' mitotic spindle and midbody, plays a role in stress fiber and filopodia formation, phagocytosis, activation of serum response factor, formation of adherens junctions, and it can act as a transcription factor. mDia1 accelerates actin nucleation and elongation by interacting with barbed ends of actin filaments. The gene encoding mDia1 is located on Chromosome 18 of Mus musculus and named Diap1.
Centralspindlin is a motor complex implicated in cell division. It contributes to virtually every step in cytokinesis, It is highly conserved in animal cells as a component of the spindle midzone and midbody. Centralspindlin is required for the assembly of the mitotic spindle as well as for microtubule bundling and anchoring of midbody microtubules to the plasma membrane. This complex is also implicated in tethering the spindle apparatus to the plasma membrane during cytokinesis This interaction permits cleavage furrow ingression. In addition, centralspindlin's interaction with the ESCRT III allows for abscission to occur.

In molecular biology, an actomyosin ring or contractile ring, is a prominent structure during cytokinesis. It forms perpendicular to the axis of the spindle apparatus towards the end of telophase, in which sister chromatids are identically separated at the opposite sides of the spindle forming nuclei. The actomyosin ring follows an orderly sequence of events: identification of the active division site, formation of the ring, constriction of the ring, and disassembly of the ring. It is composed of actin and myosin II bundles, thus the term actomyosin. The actomyosin ring operates in contractile motion, although the mechanism on how or what triggers the constriction is still an evolving topic. Other cytoskeletal proteins are also involved in maintaining the stability of the ring and driving its constriction. Apart from cytokinesis, in which the ring constricts as the cells divide, actomyosin ring constriction has also been found to activate during wound closure. During this process, actin filaments are degraded, preserving the thickness of the ring. After cytokinesis is complete, one of the two daughter cells inherits a remnant known as the midbody ring.
Edwin W. Taylor is an adjunct professor of cell and developmental biology at Northwestern University. He was elected to the National Academy of Sciences in 2001. Taylor received a BA in physics and chemistry from the University of Toronto in 1952; an MSc in physical chemistry from McMaster University in 1955, and a PhD in biophysics from the University of Chicago in 1957. In 2001 Taylor was elected to the National Academy of Scineces in Cellular and Developmental Biology and Biochemistry.


















